Geranylgeranylacetone selectively binds to the HSP70 of Helicobacter pylori and alters its coccoid morphology
- PMID: 26345206
- PMCID: PMC4561889
- DOI: 10.1038/srep13738
Geranylgeranylacetone selectively binds to the HSP70 of Helicobacter pylori and alters its coccoid morphology
Abstract
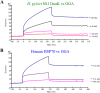
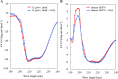
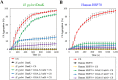
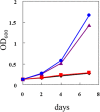
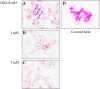
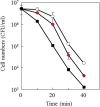
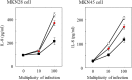
Geranylgeranylacetone (GGA) is used to treat patients suffering from peptic ulcers and gastritis. We examined the effect of GGA on Helicobacter pylori, which is a causative factor of gastrointestinal diseases. Previously, we have reported that GGA binds specifically to the molecular chaperone HSP70. In this paper, we report that GGA bounds to H. pylori HSP70 (product of the DnaK gene) with 26-times higher affinity than to human HSP70, and induced large conformational changes as observed from surface plasmon resonance and circular dichroism. Binding of GGA suppressed the activity of the H. pylori chaperone. GGA also altered several characteristics of H. pylori cells. GGA-treated cells elicited enhanced interleukin-8 production by gastric cancer cell lines and potentiated susceptibility to complement as compared to untreated cells. GGA also caused morphological alterations in H. pylori as reflected in fewer coccoid-like cells, suggesting that GGA converts H. pylori to an actively dividing, spiral state (vegetative form) from a non-growing, coccoid state. This morphological conversion by GGA resulted in accelerated growth of H. pylori. These results suggest a model in which GGA sensitizes H. pylori to antibiotic treatment by converting the cells to an actively growing state.
Figures

References
-
- Walker M. M. & Crabtree J. E. Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann. N. Y. Acad. Sci. 859, 96–111 (1998). - PubMed
-
- Israel D. A. & Peek R. M. Jr. The role of persistence in Helicobacter pylori pathogenesis. Curr. Opin. Gastroenterol. 22, 3–7 (2006). - PubMed
-
- Murakami K. et al. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 48, 1128–35 (2013). - PubMed
-
- Chan W. Y. et al. Coccoid forms of Helicobacter pylori in the human stomach. Am. J. Clin. Pathol. 102, 503–507 (1994). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

