Cilostazol inhibits plasmacytoid dendritic cell activation and antigen presentation
- PMID: 26345215
- PMCID: PMC4554787
- DOI: 10.11909/j.issn.1671-5411.2015.04.011
Cilostazol inhibits plasmacytoid dendritic cell activation and antigen presentation
Abstract
Background: Cilostazol, an anti-platelet drug for treating coronary heart disease, has been reported to modulate immune cell functions. Plasmacytoid dendritic cells (pDCs) have been found to participate in the progression of atherosclerosis mainly through interferon α (IFN-α) production. Whether cilostazol influences pDCs activation is still not clear. In this study, we aimed to investigate the effects of cilostazol on cell activation and antigen presentation of pDCs in vitro in this study.
Methods: Peripheral blood mononuclear cells isolated by Ficoll centrifugation and pDCs sorted by flow cytometry were used in this study. After pretreated with cilostazol for 2 h, cells were stimulated with CpG-A, R848 or virus for 6 h or 20 h, or stimulated with CpG-B for 48 h and then co-cultured with naïve T cell for five days. Cytokines in supernatant and intracellular cytokines were analyzed by ELISA or flow cytometry respectively.
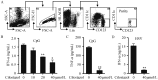
Results: Our data indicated that cilostazol could inhibit IFN-α and tumor necrosis factor α (TNF-α) production from pDCs in a dose-dependent manner. In addition, the ability of priming naïve T cells of pDCs was also impaired by cilostazol. The inhibitory effect was not due to cell killing since the viability of pDCs did not change upon cilostazol treatment.
Conclusion: Cilostazol inhibits pDCs cell activation and antigen presentation in vitro, which may explain how cilostazol protects against atherosclerosis.
Keywords: Antigen presentation; Cilostazol; Interferon α; Plasmacytoid dendritic cell; Tumor necrosis factor α.
Figures




Similar articles
-
Distinctive downmodulation of plasmacytoid dendritic cell functions by vitamin D3 analogue calcipotriol.J Dermatol Sci. 2016 Oct;84(1):71-79. doi: 10.1016/j.jdermsci.2016.06.003. Epub 2016 Jun 3. J Dermatol Sci. 2016. PMID: 27342039
-
Inhibitory effects of human immunodeficiency virus gp120 and Tat on CpG-A-induced inflammatory cytokines in plasmacytoid dendritic cells.Acta Biochim Biophys Sin (Shanghai). 2012 Sep;44(9):797-804. doi: 10.1093/abbs/gms062. Epub 2012 Jul 19. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22814248
-
Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha.J Allergy Clin Immunol. 2008 Feb;121(2):486-91. doi: 10.1016/j.jaci.2007.09.049. Epub 2007 Nov 26. J Allergy Clin Immunol. 2008. PMID: 18036648
-
Plasmacytoid dendritic cells activate allergen-specific TH2 memory cells: modulation by CpG oligodeoxynucleotides.J Allergy Clin Immunol. 2004 Aug;114(2):436-43. doi: 10.1016/j.jaci.2004.04.035. J Allergy Clin Immunol. 2004. PMID: 15316529
-
Culture supernatants of oral cancer cells induce impaired IFN-α production of pDCs partly through the down-regulation of TLR-9 expression.Arch Oral Biol. 2018 Sep;93:141-148. doi: 10.1016/j.archoralbio.2018.06.006. Epub 2018 Jun 5. Arch Oral Biol. 2018. PMID: 29913322
Cited by
-
Transcriptomic analysis reveals Cilostazol's role in ameliorating cardiovascular disease: Inhibition of monocyte-to-macrophage differentiation and reduction of endothelial cell reactive oxygen species production.Heliyon. 2024 Apr 3;10(7):e29194. doi: 10.1016/j.heliyon.2024.e29194. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38601627 Free PMC article.
-
Could cilostazol be beneficial in COVID-19 treatment? Thinking about phosphodiesterase-3 as a therapeutic target.Int Immunopharmacol. 2021 Mar;92:107336. doi: 10.1016/j.intimp.2020.107336. Epub 2020 Dec 28. Int Immunopharmacol. 2021. PMID: 33418248 Free PMC article. Review.
References
-
- Jennings DL, Kalus JS. Addition of cilostazol to aspirin and a thienopyridine for prevention of restenosis after coronary artery stenting: a meta-analysis. J Clin Pharmacol. 2010;50:415–421. - PubMed
-
- Wang S, Yan C, Xu H, et al. Suppression of encephalitogenic T-cell responses by cilostazol is associated with upregulation of regulatory T cells. Neuroreport. 2010;21:629–635. - PubMed
-
- Chuang SY, Yang SH, Pang JH. Cilostazol reduces MCP-1-induced chemotaxis and adhesion of THP-1 monocytes by inhibiting CCR2 gene expression. Biochem Biophys Res Commun. 2011;411:402–408. - PubMed
-
- Lee JH, Oh GT, Park SY, et al. Cilostazol reduces atherosclerosis by inhibition of superoxide and tumor necrosis factor-alpha formation in low-density lipoprotein receptor-null mice fed high cholesterol. J Pharmacol Exp Ther. 2005;313:502–509. - PubMed
-
- Park SY, Lee SW, Lee WS, et al. RhoA/ROCK-dependent pathway is required for TLR2-mediated IL-23 production in human synovial macrophages: suppression by cilostazol. Biochem Pharmacol. 2013;86:1320–1327. - PubMed
LinkOut - more resources
Full Text Sources
