Immunohistochemical Characterization of Facioscapulohumeral Muscular Dystrophy Muscle Biopsies
- PMID: 26345300
- PMCID: PMC4560242
- DOI: 10.3233/JND-150077
Immunohistochemical Characterization of Facioscapulohumeral Muscular Dystrophy Muscle Biopsies
Abstract
Background: Posited pathological mechanisms in Facioscapulohumeral Muscular Dystrophy (FSHD) include activation in somatic tissue of normally silenced genes, increased susceptibility to oxidative stress, and induction of apoptosis.
Objective: To determine the histopathological changes in FSHD muscle biopsies and compare to possible pathological mechanisms of disease.
Methods: We performed a cross-sectional study on quadriceps muscle biopsies from 32 genetically confirmed FSHD participants, compared to healthy volunteers and myotonic dystrophy type 1 as disease controls. Biopsies were divided into groups to evaluate apoptosis rates, capillary density, myonuclear and satellite cell counts.
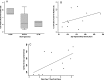
Results: Apoptosis rates were increased in FSHD (n=10, 0.74%) compared to myotonic dystrophy type 1 (n=10, 0.14%, P=0.003) and healthy volunteers (n=14, 0.13%, P=0.002). Apoptosis was higher in FSHD patients with the smallest residual D4Z4 fragments. Capillary density was decreased in FSHD1 (n=10, 316 capillaries/mm2) compared to healthy volunteers (n=15, 448 capillaries/mm2, P=0.001). No differences were seen in myonuclear or satellite cell counts.
Conclusions: Preliminary evidence for increased apoptosis rates and reduced capillary density may reflect histopathological correlates of disease activity in FSHD. The molecular-pathological correlates to these changes warrants further investigation.
Keywords: Facioscapulohumeral muscular dystrophy; apoptosis rates; capillary density; myonuclear density; satellite cell counts.
Figures

References
-
- Flanigan K.M., Coffeen C.M., Sexton L., Stauffer D., Brunner S., Leppert M.F. Genetic characterization of a large,historically significant Utah kindred with facioscapulohumeraldystrophy. Neuromuscul Disord. 2001;11:525–529. - PubMed
-
- Padberg G.W., Frants R.R., Brouwer O.F., Wijmenga C., Bakker E., Sandkuijl L.A. Facioscapulohumeral muscular dystrophy in the Dutch population. Muscle Nerve. 1995;2:S81–S84. - PubMed
-
- Celegato B., Capitanio D., Pescatori M., et al. Parallel protein and transcript profiles of FSHD patient muscles correlate to the D4Z4 arrangement and reveal a common impairment of slow to fast fibre differentiation and a general deregulation of MyoD-dependent genes. Proteomics. 2006;6:5303–5321. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

