Blood or Urine IP-10 Cannot Discriminate between Active Tuberculosis and Respiratory Diseases Different from Tuberculosis in Children
- PMID: 26346028
- PMCID: PMC4540955
- DOI: 10.1155/2015/589471
Blood or Urine IP-10 Cannot Discriminate between Active Tuberculosis and Respiratory Diseases Different from Tuberculosis in Children
Abstract
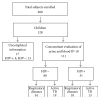
Objectives: Interferon-γ inducible protein 10 (IP-10), either in blood or in urine, has been proposed as a tuberculosis (TB) biomarker for adults. This study aims to evaluate the potential of IP-10 diagnostics in children from Uganda, a high TB-endemic country.
Methods: IP-10 was measured in the blood and urine concomitantly taken from children who were prospectively enrolled with suspected active TB, with or without HIV infection. Clinical/microbiological parameters and commercially available TB-immune assays (tuberculin skin test (TST) and QuantiFERON TB-Gold In-Tube (QFT-IT)) were concomitantly evaluated.
Results: One hundred twenty-eight children were prospectively enrolled. The analysis was performed on 111 children: 80 (72%) of them were HIV-uninfected and 31 (27.9%) were HIV-infected. Thirty-three healthy adult donors (HAD) were included as controls. The data showed that IP-10 is detectable in the urine and blood of children with active TB, independent of HIV status and age. However, although IP-10 levels were higher in active TB children compared to HAD, the accuracy of identifying "active TB" was low and similar to the TST and QFT-IT.
Conclusion: IP-10 levels are higher in children with respiratory illness compared to controls, independent of "TB status" suggesting that the evaluation of this parameter can be used as an inflammatory marker more than a TB test.
Figures


References
-
- WHO. Global tuberculosis report 2014. WHO Global TB Report 2014. 2014
-
- Portevin D., Moukambi F., Clowes P., et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. The Lancet Infectious Diseases. 2014;14(10):931–938. doi: 10.1016/s1473-3099(14)70884-9. - DOI - PubMed
-
- Cuevas L. E., Browning R., Bossuyt P., et al. Evaluation of tuberculosis diagnostics in children: 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. Consensus from an expert panel. The Journal of Infectious Diseases. 2012;205(supplement 2):S209–S215. doi: 10.1093/infdis/jir879. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

