Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV Vaccine Trial
- PMID: 26346155
- PMCID: PMC4561367
- DOI: 10.1136/bmj.h4358
Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV Vaccine Trial
Abstract
Objective: To examine the effect of the bivalent human papillomavirus (HPV) vaccine on miscarriage.
Design: Observational long term follow-up of a randomized, double blinded trial combined with an independent unvaccinated population based cohort.
Setting: Single center study in Costa Rica.
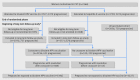
Participants: 7466 women in the trial and 2836 women in the unvaccinated cohort enrolled at the end of the randomized trial and in parallel with the observational trial component.
Intervention: Women in the trial were assigned to receive three doses of bivalent HPV vaccine (n=3727) or the control hepatitis A vaccine (n=3739). Crossover bivalent HPV vaccination occurred in the hepatitis A vaccine arm at the end of the trial. Women in the unvaccinated cohort received (n=2836) no vaccination.
Main outcome measure: Risk of miscarriage, defined by the US Centers for Disease Control and Prevention as fetal loss within 20 weeks of gestation, in pregnancies exposed to bivalent HPV vaccination in less than 90 days and any time from vaccination compared with pregnancies exposed to hepatitis A vaccine and pregnancies in the unvaccinated cohort.
Results: Of 3394 pregnancies conceived at any time since bivalent HPV vaccination, 381 pregnancies were conceived less than 90 days from vaccination. Unexposed pregnancies comprised 2507 pregnancies conceived after hepatitis A vaccination and 720 conceived in the unvaccinated cohort. Miscarriages occurred in 451 (13.3%) of all exposed pregnancies, in 50 (13.1%) of the pregnancies conceived less than 90 days from bivalent HPV vaccination, and in 414 (12.8%) of the unexposed pregnancies, of which 316 (12.6%) were in the hepatitis A vaccine group and 98 (13.6%) in the unvaccinated cohort. The relative risk of miscarriage for pregnancies conceived less than 90 days from vaccination compared with all unexposed pregnancies was 1.02 (95% confidence interval 0.78 to 1.34, one sided P=0.436) in unadjusted analyses. Results were similar after adjusting for age at vaccination (relative risk 1.15, one sided P=0.17), age at conception (1.03, P=0.422), and calendar year (1.06, P=0.358), and in stratified analyses. Among pregnancies conceived at any time from bivalent HPV vaccination, exposure was not associated with an increased risk of miscarriage overall or in subgroups, except for miscarriages at weeks 13-20 of gestation (relative risk 1.35, 95% confidence interval 1.02 to 1.77, one sided P=0.017).
Conclusions: There is no evidence that bivalent HPV vaccination affects the risk of miscarriage for pregnancies conceived less than 90 days from vaccination. The increased risk estimate for miscarriages in a subgroup of pregnancies conceived any time after vaccination may be an artifact of a thorough set of sensitivity analyses, but since a genuine association cannot totally be ruled out, this signal should nevertheless be explored further in existing and future studies.Trial registration Clinicaltrials.gov NCT00128661 and NCT01086709.
© Panagiotou et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

Comment in
-
HPV vaccination and pregnancy.BMJ. 2015 Sep 7;351:h4705. doi: 10.1136/bmj.h4705. BMJ. 2015. PMID: 26346481 No abstract available.
References
-
- Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010;59:626-9. - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:1-30. - PubMed
-
- Kuehn BM. Immunization recommendations expanded for hepatitis B, HPV, pertussis vaccines. JAMA 2012;307:1353-4. - PubMed
-
- Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine 2014;32:2670-4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
