Probing cytochrome c in living mitochondria with surface-enhanced Raman spectroscopy
- PMID: 26346634
- PMCID: PMC4561893
- DOI: 10.1038/srep13793
Probing cytochrome c in living mitochondria with surface-enhanced Raman spectroscopy
Abstract
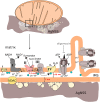
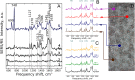
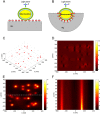
Selective study of the electron transport chain components in living mitochondria is essential for fundamental biophysical research and for the development of new medical diagnostic methods. However, many important details of inter- and intramembrane mitochondrial processes have remained in shadow due to the lack of non-invasive techniques. Here we suggest a novel label-free approach based on the surface-enhanced Raman spectroscopy (SERS) to monitor the redox state and conformation of cytochrome c in the electron transport chain in living mitochondria. We demonstrate that SERS spectra of living mitochondria placed on hierarchically structured silver-ring substrates provide exclusive information about cytochrome c behavior under modulation of inner mitochondrial membrane potential, proton gradient and the activity of ATP-synthetase. Mathematical simulation explains the observed enhancement of Raman scattering due to high concentration of electric near-field and large contact area between mitochondria and nanostructured surfaces.
Figures


References
-
- Nicholls D. G. & Ferguson S. J. Bioenergetics. (Ed. Waltham M. A.). (Academic Press Elsevier Science Ltd., 2002).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

