A Zinc(II) Photocage Based on a Decarboxylation Metal Ion Release Mechanism for Investigating Homeostasis and Biological Signaling
- PMID: 26346802
- PMCID: PMC4615554
- DOI: 10.1002/anie.201505778
A Zinc(II) Photocage Based on a Decarboxylation Metal Ion Release Mechanism for Investigating Homeostasis and Biological Signaling
Abstract
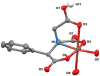
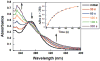
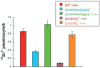
Metal ion signaling in biology has been studied extensively with ortho-nitrobenzyl photocages; however, the low quantum yields and other optical properties are not ideal for these applications. We describe the synthesis and characterization of NTAdeCage, the first member in a new class of Zn(2+) photocages that utilizes a light-driven decarboxylation reaction in the metal ion release mechanism. NTAdeCage binds Zn(2+) with sub-pM affinity using a modified nitrilotriacetate chelator and exhibits an almost 6 order of magnitude decrease in metal binding affinity upon uncaging. In contrast to other metal ion photocages, NTAdeCage and the corresponding Zn(2+) complex undergo efficient photolysis with quantum yields approaching 30 %. The ability of NTAdeCage to mediate the uptake of (65) Zn(2+) by Xenopus laevis oocytes expressing hZIP4 demonstrates the viability of this photocaging strategy to execute biological assays.
Keywords: X-ray diffraction; cage compounds; coordination compounds; photolysis; zinc.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
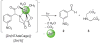

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

