Expression Pattern of Fatty Acid Binding Proteins in Celiac Disease Enteropathy
- PMID: 26346822
- PMCID: PMC4540995
- DOI: 10.1155/2015/738563
Expression Pattern of Fatty Acid Binding Proteins in Celiac Disease Enteropathy
Abstract
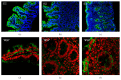
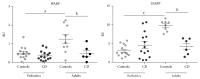
Celiac disease (CD) is an immune-mediated enteropathy that develops in genetically susceptible individuals following exposure to dietary gluten. Severe changes at the intestinal mucosa observed in untreated CD patients are linked to changes in the level and in the pattern of expression of different genes. Fully differentiated epithelial cells express two isoforms of fatty acid binding proteins (FABPs): intestinal and liver, IFABP and LFABP, respectively. These proteins bind and transport long chain fatty acids and also have other important biological roles in signaling pathways, particularly those related to PPARγ and inflammatory processes. Herein, we analyze the serum levels of IFABP and characterize the expression of both FABPs at protein and mRNA level in small intestinal mucosa in severe enteropathy and normal tissue. As a result, we observed higher levels of circulating IFABP in untreated CD patients compared with controls and patients on gluten-free diet. In duodenal mucosa a differential FABPs expression pattern was observed with a reduction in mRNA levels compared to controls explained by the epithelium loss in severe enteropathy. In conclusion, we report changes in FABPs' expression pattern in severe enteropathy. Consequently, there might be alterations in lipid metabolism and the inflammatory process in the small intestinal mucosa.
Figures




Similar articles
-
Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine.Prostaglandins Leukot Essent Fatty Acids. 2015 Feb;93:9-16. doi: 10.1016/j.plefa.2014.10.001. Epub 2014 Oct 14. Prostaglandins Leukot Essent Fatty Acids. 2015. PMID: 25458898 Free PMC article. Review.
-
Expression of microbiota, Toll-like receptors, and their regulators in the small intestinal mucosa in celiac disease.J Pediatr Gastroenterol Nutr. 2012 Jun;54(6):727-32. doi: 10.1097/MPG.0b013e318241cfa8. J Pediatr Gastroenterol Nutr. 2012. PMID: 22134550
-
Peroxisome proliferator-activated receptor-γ and thymic stromal lymphopoietin are involved in the pathophysiology of childhood coeliac disease.Virchows Arch. 2014 Oct;465(4):385-93. doi: 10.1007/s00428-014-1650-2. Epub 2014 Sep 4. Virchows Arch. 2014. PMID: 25187315
-
The proximal intestinal Fatty Acid-Binding Proteins liver FABP (LFABP) and intestinal FABP (IFABP) differentially modulate whole body energy homeostasis but are not centrally involved in net dietary lipid absorption: Studies of the LFABP/IFABP double knockout mouse.Biochim Biophys Acta Mol Cell Biol Lipids. 2023 Jan;1868(1):159238. doi: 10.1016/j.bbalip.2022.159238. Epub 2022 Oct 4. Biochim Biophys Acta Mol Cell Biol Lipids. 2023. PMID: 36206853 Free PMC article.
-
Emerging role of fatty acid binding proteins in cancer pathogenesis.Histol Histopathol. 2019 Jan;34(1):1-12. doi: 10.14670/HH-18-014. Epub 2018 Jun 18. Histol Histopathol. 2019. PMID: 29911740 Review.
Cited by
-
HIF1α-Regulated Expression of the Fatty Acid Binding Protein Family Is Important for Hypoxic Reactivation of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2021 May 24;95(12):e02063-20. doi: 10.1128/JVI.02063-20. Print 2021 May 24. J Virol. 2021. PMID: 33789996 Free PMC article.
-
Blood Biomarkers of Intestinal Epithelium Damage Regenerating Islet-derived Protein 3α and Trefoil Factor 3 Are Persistently Elevated in Patients with Alcoholic Hepatitis.Alcohol Clin Exp Res. 2021 Apr;45(4):720-731. doi: 10.1111/acer.14579. Epub 2021 Mar 11. Alcohol Clin Exp Res. 2021. PMID: 33587293 Free PMC article.
-
The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development.Front Oncol. 2021 Apr 15;11:626349. doi: 10.3389/fonc.2021.626349. eCollection 2021. Front Oncol. 2021. PMID: 33937029 Free PMC article. Review.
-
Typing of HLA susceptibility alleles as complementary tool in diagnosis of controversial cases of pediatric celiac disease.Front Nutr. 2025 Feb 25;12:1500632. doi: 10.3389/fnut.2025.1500632. eCollection 2025. Front Nutr. 2025. PMID: 40070485 Free PMC article.
-
sCD14 and Intestinal Fatty Acid Binding Protein Are Elevated in the Serum of Patients With Idiopathic Anaphylaxis.J Allergy Clin Immunol Pract. 2023 Jul;11(7):2080-2086.e5. doi: 10.1016/j.jaip.2023.03.037. Epub 2023 Mar 29. J Allergy Clin Immunol Pract. 2023. PMID: 36997122 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

