Artificial Molecular Machines
- PMID: 26346838
- PMCID: PMC4585175
- DOI: 10.1021/acs.chemrev.5b00146
Artificial Molecular Machines
Figures
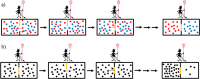
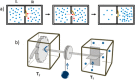
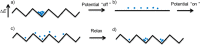
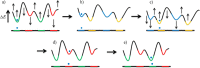


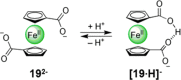
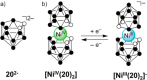
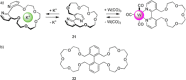
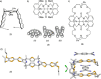
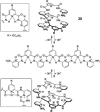
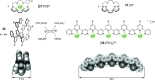
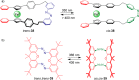
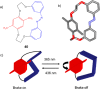
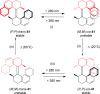
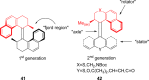
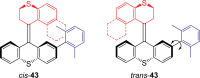
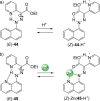
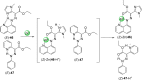

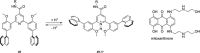

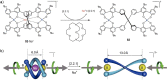
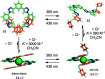

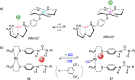
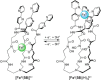

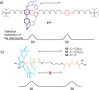
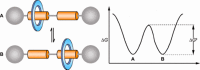
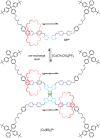
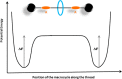
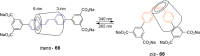
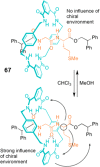
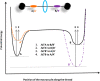
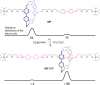
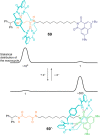
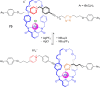
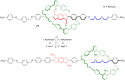

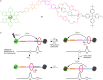
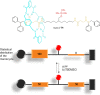
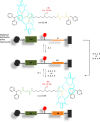
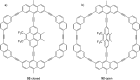
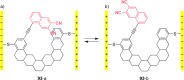
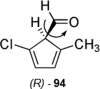
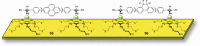


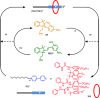
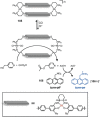
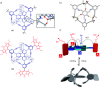

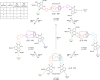
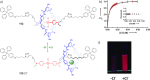

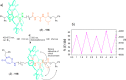

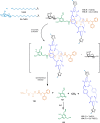
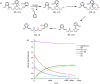


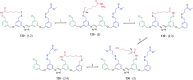
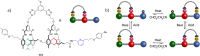
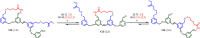
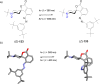
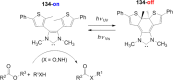
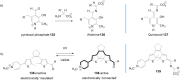
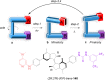
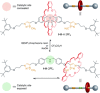



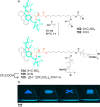

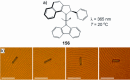
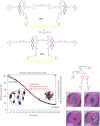
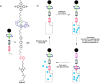

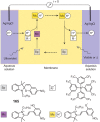
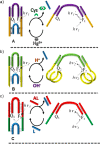
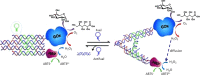
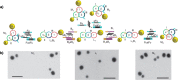

References
-
- Brown R. A Brief Account of Microscopical Observations Made on the Particles Contained in the Pollen of Plants. Philos. Mag. 1828, 4, 171–17310.1080/14786442808674769. - DOI
-
- Brown R. On the Particles Contained in the Pollen of Plants; and on the General Existence of Active Molecules in Organic and Inorganic Bodies. Edinb. New Philos. J. 1828, 5, 358–371.
-
- Perrin J. In Atoms (English Translation), 2nd ed.; Hammick D. L., Ed.; Constable and Co.: London, 1923.
-
- Einstein A. Über die von der Molekularkinetischen Theorie der Wärme Geforderte Bewegung von in Ruhenden Flüssigkeiten Suspendierten Teilchen. Ann. Phys. 1905, 17, 549–56010.1002/andp.19053220806. - DOI
-
- Feynman R. P.; Leighton R. B.; Sands M.. The Feynman Lectures on Physics; Addison-Wesley: Reading, MA, 1963; Vol. 1, Chapter 46.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

