The anti-inflammatory and antifibrotic effects of Coreopsis tinctoria Nutt on high-glucose-fat diet and streptozotocin-induced diabetic renal damage in rats
- PMID: 26346939
- PMCID: PMC4561427
- DOI: 10.1186/s12906-015-0826-x
The anti-inflammatory and antifibrotic effects of Coreopsis tinctoria Nutt on high-glucose-fat diet and streptozotocin-induced diabetic renal damage in rats
Abstract
Background: Diabetic nephropathy is a serious complication of diabetes whose development process is associated with inflammation, renal hypertrophy, and fibrosis. Coreopsis tinctoria Nutt, traditionally used as a healthcare tea, has anti-inflammatory, anti-hyperlipidemia, and glycemic regulation activities. The aim of our study was to investigate the renal protective effect of ethyl acetate extract of C. tinctoria Nutt (AC) on high-glucose-fat diet and streptozotocin (STZ)-induced diabetic rats.
Methods: A diabetic rat model was induced by high-glucose-fat diet and intraperitoneal injection of 35 mg/kg STZ. After treatment with AC at a daily dose of 150, 300 or, 600 mg/kg for 4 weeks, metabolic and renal function parameters of serum and urine were examined. Degree of renal damage, renal proinflammatory cytokines, and fibrotic protein expression were analyzed by histopathology and immunohistochemistry. Renal AMP-activated protein kinase (AMPK) and transforming growth factor (TGF)-β1/Smad signaling pathway were determined by western blotting.
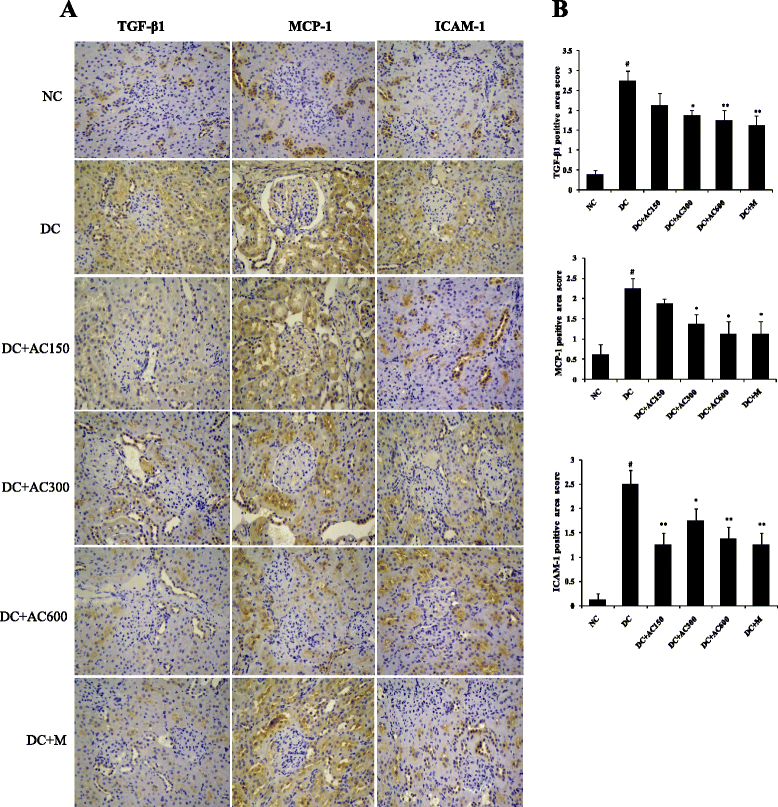
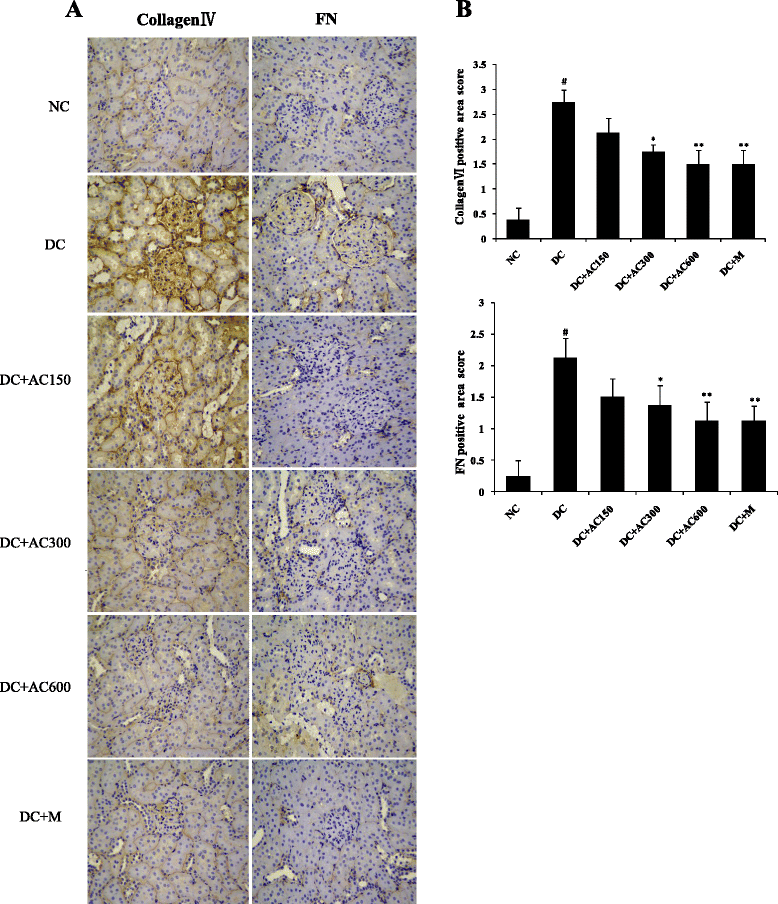
Results: Diabetic rats showed obvious renal dysfunction, inflammation and fibrosis. However, AC significantly reduced levels of blood glucose, total cholesterol, triglyceride, blood urea nitrogen, serum creatinine and urinary albumin, as well as expression of kidney proinflammatory cytokines of monocyte chemoattractant protein-1 and intercellular adhesion molecule-1. AC also ameliorated renal hypertrophy and fibrosis by reducing fibronectin and collagen IV and suppressing the TGF-β1/Smad signaling pathway. Meanwhile, AMPKα as a protective cytokine was markedly stimulated by AC.
Conclusion: In summary, AC controls blood glucose, inhibits inflammatory and fibrotic processes, suppresses the TGF-β1/Smad signaling pathway, and activates phosphorylation of AMPKα in the kidneys, which confirms the protective effects of AC in the early stage of diabetic kidney disease.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

