Clinical potential of vorapaxar in cardiovascular risk reduction in patients with atherosclerosis
- PMID: 26346960
- PMCID: PMC4529257
- DOI: 10.2147/TCRM.S55469
Clinical potential of vorapaxar in cardiovascular risk reduction in patients with atherosclerosis
Abstract
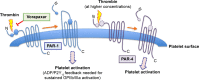
Vorapaxar (ZONTIVITY™, formerly known as SCH 530348) is a specific, orally active antagonist of the protease-activated receptor-1 (PAR-1) on platelets. It inhibits thrombin-induced platelet activation by binding to the ectodomain of PAR-1. After animal studies and Phase II studies showed that vorapaxar sufficiently inhibits platelet activation without significantly increasing bleeding complications, safety and efficacy of vorapaxar were assessed in two large multicenter trials in patients with coronary artery disease and atherosclerosis. The Thrombin-Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndromes (TRACER) trial investigated safety and efficacy of vorapaxar in patients with an acute coronary syndrome without ST-segment elevation. The Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-Thrombolysis In Myocardial Infarction 50 (TRA 2°P-TIMI 50) investigated atherothrombotic events in patients with stable atherosclerosis. Results of both studies suggested that vorapaxar given in addition to standard antiplatelet therapy can reduce atherothrombotic events, but increases the risk of mild and moderate bleeding complications. This review article summarizes the main results of TRACER and TRA 2°P-TIMI 50 and suggests patient cohorts that might benefit from treatment with vorapaxar in addition to standard antiplatelet therapy.
Keywords: antiplatelet therapy; atherosclerosis; myocardial infarction; vorapaxar.
Figures
References
-
- Heeschen C, Dimmeler S, Hamm CW, et al. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–1111. - PubMed
-
- Royle NJ, Irwin DM, Koschinsky ML, MacGillivray RT, Hamerton JL. Human genes encoding prothrombin and ceruloplasmin map to 11p11-q12 and 3q21-24, respectively. Somat Cell Mol Genet. 1987;13:285–292. - PubMed
-
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. - PubMed
-
- Cook JJ, Sitko GR, Bednar B, et al. An antibody against the exosite of the cloned thrombin receptor inhibits experimental arterial thrombosis in the African green monkey. Circulation. 1995;91:2961–2971. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

