A bioinformatic and mechanistic study elicits the antifibrotic effect of ursolic acid through the attenuation of oxidative stress with the involvement of ERK, PI3K/Akt, and p38 MAPK signaling pathways in human hepatic stellate cells and rat liver
- PMID: 26347199
- PMCID: PMC4529259
- DOI: 10.2147/DDDT.S85426
A bioinformatic and mechanistic study elicits the antifibrotic effect of ursolic acid through the attenuation of oxidative stress with the involvement of ERK, PI3K/Akt, and p38 MAPK signaling pathways in human hepatic stellate cells and rat liver
Abstract
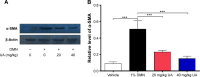
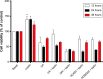
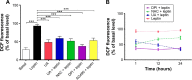
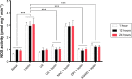
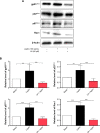
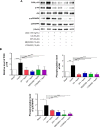
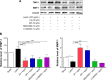
NADPH oxidases (NOXs) are a predominant mediator of redox homeostasis in hepatic stellate cells (HSCs), and oxidative stress plays an important role in the pathogenesis of liver fibrosis. Ursolic acid (UA) is a pentacyclic triterpenoid with various pharmacological activities, but the molecular targets and underlying mechanisms for its antifibrotic effect in the liver remain elusive. This study aimed to computationally predict the molecular interactome and mechanistically investigate the antifibrotic effect of UA on oxidative stress, with a focus on NOX4 activity and cross-linked signaling pathways in human HSCs and rat liver. Drug-drug interaction via chemical-protein interactome tool, a server that can predict drug-drug interaction via chemical-protein interactome, was used to predict the molecular targets of UA, and Database for Annotation, Visualization, and Integrated Discovery was employed to analyze the signaling pathways of the predicted targets of UA. The bioinformatic data showed that there were 611 molecular proteins possibly interacting with UA and that there were over 49 functional clusters responding to UA. The subsequential benchmarking data showed that UA significantly reduced the accumulation of type I collagen in HSCs in rat liver, increased the expression level of MMP-1, but decreased the expression level of TIMP-1 in HSC-T6 cells. UA also remarkably reduced the gene expression level of type I collagen in HSC-T6 cells. Furthermore, UA remarkably attenuated oxidative stress via negative regulation of NOX4 activity and expression in HSC-T6 cells. The employment of specific chemical inhibitors, SB203580, LY294002, PD98059, and AG490, demonstrated the involvement of ERK, PI3K/Akt, and p38 MAPK signaling pathways in the regulatory effect of UA on NOX4 activity and expression. Collectively, the antifibrotic effect of UA is partially due to the oxidative stress attenuating effect through manipulating NOX4 activity and expression. The results suggest that UA may act as a promising antifibrotic agent. More studies are warranted to evaluate the safety and efficacy of UA in the treatment of liver fibrosis.
Keywords: DAVID; DDI-CPI; NADPH oxidase; ROS; liver fibrosis; ursolic acid.
Figures




Similar articles
-
Gambogic acid attenuates liver fibrosis by inhibiting the PI3K/AKT and MAPK signaling pathways via inhibiting HSP90.Toxicol Appl Pharmacol. 2019 May 15;371:63-73. doi: 10.1016/j.taap.2019.03.028. Epub 2019 Apr 3. Toxicol Appl Pharmacol. 2019. PMID: 30953615
-
Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways.Liver Int. 2015 Apr;35(4):1222-33. doi: 10.1111/liv.12638. Epub 2014 Aug 5. Liver Int. 2015. PMID: 25040634
-
NP603, a novel and potent inhibitor of FGFR1 tyrosine kinase, inhibits hepatic stellate cell proliferation and ameliorates hepatic fibrosis in rats.Am J Physiol Cell Physiol. 2011 Aug;301(2):C469-77. doi: 10.1152/ajpcell.00452.2010. Epub 2011 May 4. Am J Physiol Cell Physiol. 2011. PMID: 21543745
-
Promising Anticancer Activities of Alismatis rhizome and Its Triterpenes via p38 and PI3K/Akt/mTOR Signaling Pathways.Nutrients. 2021 Jul 18;13(7):2455. doi: 10.3390/nu13072455. Nutrients. 2021. PMID: 34371964 Free PMC article. Review.
-
Role of NADPH oxidases in liver fibrosis.Antioxid Redox Signal. 2014 Jun 10;20(17):2854-72. doi: 10.1089/ars.2013.5619. Epub 2014 Jan 24. Antioxid Redox Signal. 2014. PMID: 24040957 Free PMC article. Review.
Cited by
-
Sorafenib effect on liver neoplastic changes in rats: more than a kinase inhibitor.Clin Exp Med. 2017 May;17(2):185-191. doi: 10.1007/s10238-016-0416-3. Epub 2016 Apr 16. Clin Exp Med. 2017. PMID: 27085325
-
Hepatoprotective Mechanisms Induced by Spinach Methanolic Extract in Rats with Hyperglycemia-An Immunohistochemical Analysis.Antioxidants (Basel). 2023 Nov 17;12(11):2013. doi: 10.3390/antiox12112013. Antioxidants (Basel). 2023. PMID: 38001866 Free PMC article.
-
Active Ingredients and Action Mechanisms of Yi Guan Jian Decoction in Chronic Hepatitis B Patients with Liver Fibrosis.Evid Based Complement Alternat Med. 2019 Sep 3;2019:2408126. doi: 10.1155/2019/2408126. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31565062 Free PMC article.
-
Ursolic acid isolated from Isodon excisoides induces apoptosis and inhibits invasion of GBC-SD gallbladder carcinoma cells.Oncol Lett. 2019 Aug;18(2):1467-1474. doi: 10.3892/ol.2019.10397. Epub 2019 May 27. Oncol Lett. 2019. PMID: 31423212 Free PMC article.
-
The use of Xuanbai Chengqi decoction on monkeypox disease through the estrone-target AR interaction.Front Microbiol. 2023 Sep 20;14:1234817. doi: 10.3389/fmicb.2023.1234817. eCollection 2023. Front Microbiol. 2023. PMID: 37808322 Free PMC article.
References
-
- Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–117. - PubMed
-
- Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51(3):729–733. - PubMed
-
- Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):e1–e6. - PubMed
-
- Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369(9573):1582–1583. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

