Fractalkine Signaling and Microglia Functions in the Developing Brain
- PMID: 26347402
- PMCID: PMC4539507
- DOI: 10.1155/2015/689404
Fractalkine Signaling and Microglia Functions in the Developing Brain
Abstract
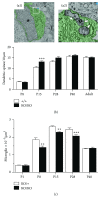
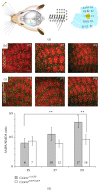
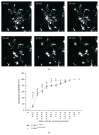
Microglial cells are the resident macrophages of the central nervous system (CNS). Besides their classical roles in pathological conditions, these immune cells also dynamically interact with neurons and influence their structure and function in physiological conditions. The neuronal chemokine fractalkine and its microglial receptor CX3CR1 are one important signaling pathway involved in these reciprocal interactions. In the present review, we will discuss recent evidence indicating that fractalkine signaling also determines several functions of microglial cells during normal CNS development. It has been known for a decade that microglial cells influence the neuronal death that normally occurs during CNS development. Surprisingly, recent evidence indicates that they can also support survival of developing neurons, control axon outgrowth, and laminar positioning of subsets of interneurons in the forebrain. Moreover, microglial cells influence the maturation of synaptic circuits at early postnatal stages: their phagocytic activity allows them to eliminate inappropriate synapses and they can also influence the functional expression of synaptic proteins by releasing mediators. Fractalkine signaling controls these functions of microglial cells in part by regulating their timely recruitment at sites of developing synapses. Finally, on-going research suggests that this signaling pathway is also a key player in neurodevelopmental disorders.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

