At the frontier of epigenetics of brain sex differences
- PMID: 26347630
- PMCID: PMC4543874
- DOI: 10.3389/fnbeh.2015.00221
At the frontier of epigenetics of brain sex differences
Abstract
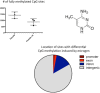
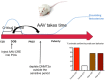
The notion that epigenetics may play an important role in the establishment and maintenance of sex differences in the brain has garnered great enthusiasm but the reality in terms of actual advances has been slow. Two general approaches include the comparison of a particular epigenetic mark in males vs. females and the inhibition of key epigenetic enzymes or co-factors to determine if this eliminates a particular sex difference in brain or behavior. The majority of emphasis has been on candidate genes such as steroid receptors. Only recently have more generalized survey type approaches been achieved and these promise to open new vistas and accelerate discovery of important roles for DNA methylation, histone modification, genomic imprinting and microRNAs (miRs). Technical challenges abound and, while not unique to this field, will require novel thinking and new approaches by behavioral neuroendocrinologists.
Keywords: amygdala; estrogens; preoptic area; reproductive behavior.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

