Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders
- PMID: 26347657
- PMCID: PMC4541029
- DOI: 10.3389/fphys.2015.00230
Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders
Abstract
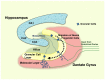
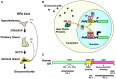
The hypothalamic-pituitary-adrenal (HPA) axis and its end-effectors glucocorticoid hormones play central roles in the adaptive response to numerous stressors that can be either internal or external. Thus, this system has a strong impact on the brain hippocampus and its major functions, such as cognition, memory as well as behavior, and mood. The hippocampal area of the adult brain contains neural stem cells or more committed neural progenitor cells, which retain throughout the human life the ability of self-renewal and to differentiate into multiple neural cell lineages, such as neurons, astrocytes, and oligodendrocytes. Importantly, these characteristic cells contribute significantly to the above-indicated functions of the hippocampus, while various stressors and glucocorticoids influence proliferation, differentiation, and fate of these cells. This review offers an overview of the current understanding on the interactions between the HPA axis/glucocorticoid stress-responsive system and hippocampal neural progenitor cells by focusing on the actions of glucocorticoids. Also addressed is a further discussion on the implications of such interactions to the pathophysiology of mood disorders.
Keywords: dentate gyrus; glucocorticoid receptor; hippocampus; major depression; neural stem cells (NSCs).
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

