DAMPs from Cell Death to New Life
- PMID: 26347745
- PMCID: PMC4539554
- DOI: 10.3389/fimmu.2015.00422
DAMPs from Cell Death to New Life
Abstract
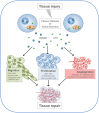
Our body handles tissue damage by activating the immune system in response to intracellular molecules released by injured tissues [damage-associated molecular patterns (DAMPs)], in a similar way as it detects molecular motifs conserved in pathogens (pathogen-associated molecular patterns). DAMPs are molecules that have a physiological role inside the cell, but acquire additional functions when they are exposed to the extracellular environment: they alert the body about danger, stimulate an inflammatory response, and finally promote the regeneration process. Beside their passive release by dead cells, some DAMPs can be secreted or exposed by living cells undergoing a life-threatening stress. DAMPs have been linked to inflammation and related disorders: hence, inhibition of DAMP-mediated inflammatory responses is a promising strategy to improve the clinical management of infection- and injury-elicited inflammatory diseases. However, it is important to consider that DAMPs are not only danger signals but also central players in tissue repair. Indeed, some DAMPs have been studied for their role in tissue healing after sterile or infection-associated inflammation. This review is focused on two exemplary DAMPs, HMGB1 and adenosine triphosphate, and their contribution to both inflammation and tissue repair.
Keywords: ATP; DAMP; HMGB1; inflammation; tissue repair.
Figures


References
-
- Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, et al. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation (1994) 57:211–7. 10.1097/00007890-199401001-00010 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

