Precise repair of mPing excision sites is facilitated by target site duplication derived microhomology
- PMID: 26347803
- PMCID: PMC4561436
- DOI: 10.1186/s13100-015-0046-4
Precise repair of mPing excision sites is facilitated by target site duplication derived microhomology
Abstract
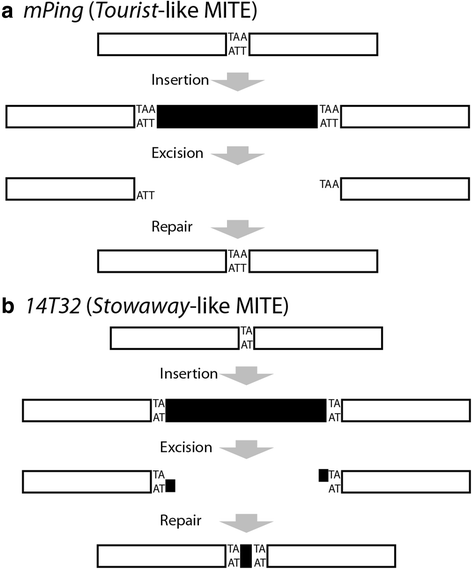
Background: A key difference between the Tourist and Stowaway families of miniature inverted repeat transposable elements (MITEs) is the manner in which their excision alters the genome. Upon excision, Stowaway-like MITEs and the associated Mariner elements usually leave behind a small duplication and short sequences from the end of the element. These small insertions or deletions known as "footprints" can potentially disrupt coding or regulatory sequences. In contrast, Tourist-like MITEs and the associated PIF/Pong/Harbinger elements generally excise precisely, returning the genome to its original state. The purpose of this study was to determine the mechanisms underlying these excision differences, including the role of the host DNA repair mechanisms.
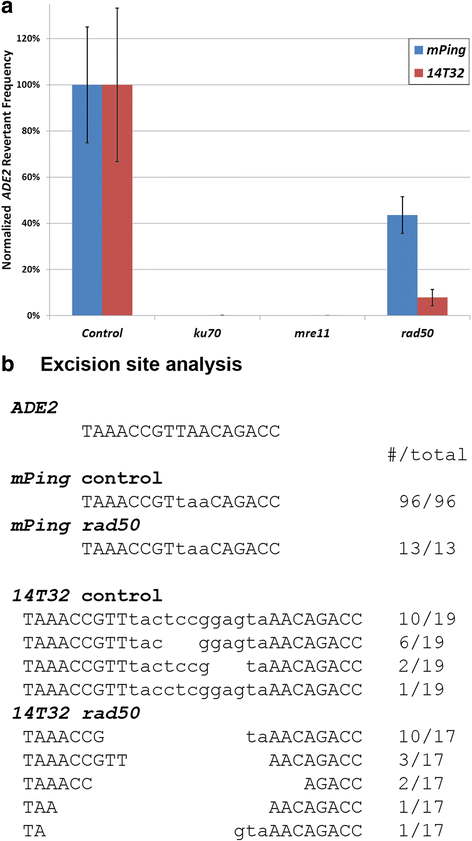
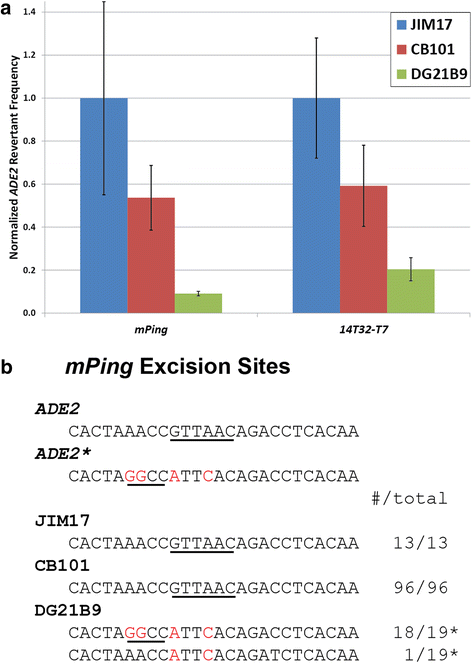
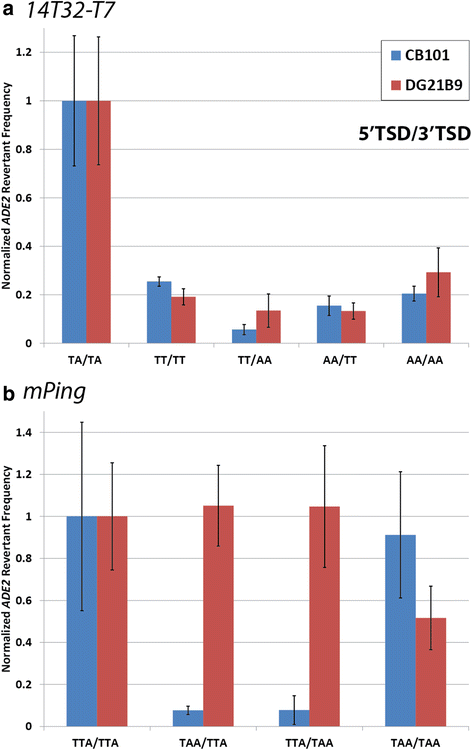
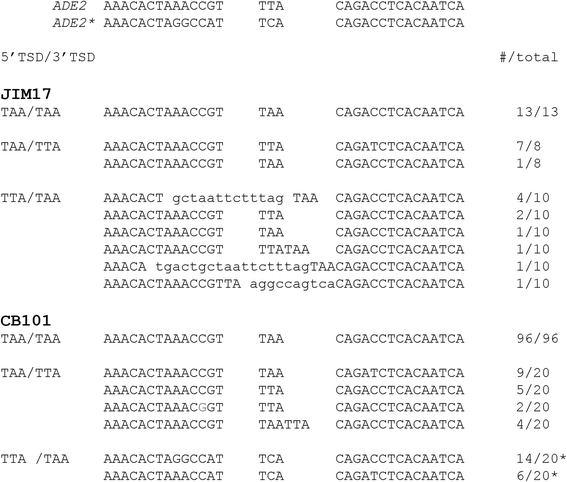
Results: The transposition of the Tourist-like element, mPing, and the Stowaway-like element, 14T32, were evaluated using yeast transposition assays. Assays performed in yeast strains lacking non-homologous end joining (NHEJ) enzymes indicated that the excision sites of both elements were primarily repaired by NHEJ. Altering the target site duplication (TSD) sequences that flank these elements reduced the transposition frequency. Using yeast strains with the ability to repair the excision site by homologous repair showed that some TSD changes disrupt excision of the element. Changing the ends of mPing to produce non-matching TSDs drastically reduced repair of the excision site and resulted in increased generation of footprints.
Conclusions: Together these results indicate that the difference in Tourist and Stowaway excision sites results from transposition mechanism characteristics. The TSDs of both elements play a role in element excision, but only the mPing TSDs actively participate in excision site repair. Our data suggests that Tourist-like elements excise with staggered cleavage of the TSDs, which provides microhomology that facilitates precise repair. This slight modification in the transposition mechanism results in more efficient repair of the double stranded break, and thus, may be less harmful to host genomes by disrupting fewer genes.
Keywords: Excision site repair; Target site duplication; mPing.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

