Review of Restricted Experiment Requests, Division of Select Agents and Toxins, Centers for Disease Control and Prevention, 2006-2013
- PMID: 26347984
- PMCID: PMC4582684
- DOI: 10.1089/hs.2015.0021
Review of Restricted Experiment Requests, Division of Select Agents and Toxins, Centers for Disease Control and Prevention, 2006-2013
Abstract
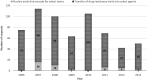
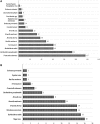
The Centers for Disease Control and Prevention (CDC) Division of Select Agents and Toxins (DSAT) regulates laboratories that possess, use, or transfer select agents and toxins in the United States. DSAT also mitigates biosafety risks through the review of "restricted experiments," which under the select agent regulations are experiments that pose heightened biosafety risks. From January 2006 through December 2013, DSAT received 618 requests from 109 entities to perform potentially restricted experiments. Of these requests, 85% were determined not to meet the regulatory definition of a restricted experiment, while 15% of the requests met the definition of a restricted experiment. Of the 91 restricted experiments proposed, DSAT approved 31 (34%) requests because the biosafety conditions proposed were commensurate with the experiments' biosafety risk. All 31 approved restricted experiments were for work with select toxins. DSAT did not approve 60 restricted experiment requests due to potentially serious biosafety risks to public health and safety. All 60 denied restricted experiments proposed inserting drug resistance traits into select agents that could compromise the control of disease. The select agents and toxins associated most frequently with requests that met the regulatory definition of a restricted experiment are Shiga toxin (n = 16), Burkholderia mallei (n = 15), Botulinum neurotoxin (n = 14), and Brucella abortus (n = 14). In general, all restricted experiment decisions are determined on a case-by-case basis. This article describes the trends and characteristics of the data associated with restricted experiment requests among select agents that have an impact on public health and safety (HHS only agents) or both public health and safety and animal health or products (overlap agents). The information presented here, coupled with the information published in the restricted experiment guidance document ( www.selectagents.gov ), is intended to promote awareness among the research community of the type of experiments that meet the regulatory definition of a restricted experiment as well as to provide a greater understanding of the restricted experiment review process.
Figures



Similar articles
-
Review of Restricted Experiment Requests, Division of Select Agents and Toxins, US Centers for Disease Control and Prevention, 2014-2021.Health Secur. 2023 May-Jun;21(3):207-213. doi: 10.1089/hs.2022.0155. Epub 2023 May 16. Health Secur. 2023. PMID: 37195716 Free PMC article. Review.
-
Possession, Use, and Transfer of Select Agents and Toxins; Biennial Review of the List of Select Agents and Toxins and Enhanced Biosafety Requirements. Final rule.Fed Regist. 2017 Jan 19;82(12):6278-94. Fed Regist. 2017. PMID: 28106357
-
Possession, use, and transfer of select agents and toxins. Final rule.Fed Regist. 2005 Mar 18;70(52):13293-325. Fed Regist. 2005. PMID: 15776528
-
Possession, use, and transfer of select agents and toxins; biennial review. Final rule.Fed Regist. 2012 Oct 5;77(194):61083-115. Fed Regist. 2012. PMID: 23038847
-
Clinical laboratories, the select agent program, and biological surety (biosurety).Clin Lab Med. 2006 Jun;26(2):299-312, vii. doi: 10.1016/j.cll.2006.03.004. Clin Lab Med. 2006. PMID: 16815454 Review.
Cited by
-
Review of Restricted Experiment Requests, Division of Select Agents and Toxins, US Centers for Disease Control and Prevention, 2014-2021.Health Secur. 2023 May-Jun;21(3):207-213. doi: 10.1089/hs.2022.0155. Epub 2023 May 16. Health Secur. 2023. PMID: 37195716 Free PMC article. Review.
-
Brucella melitensis Wzm/Wzt System: Changes in the Bacterial Envelope Lead to Improved Rev1Δwzm Vaccine Properties.Front Microbiol. 2022 Jul 4;13:908495. doi: 10.3389/fmicb.2022.908495. eCollection 2022. Front Microbiol. 2022. PMID: 35875565 Free PMC article.
References
-
- Select Agent Regulation. 42 CFR Part 73: Possession, Use, and Transfer of Select Agents and Toxins; Final Rule. Fed Regist March 18, 2005. https://oig.hhs.gov/authorities/docs/05/032905FRselectagents.pdf Accessed August19, 2015
-
- Select Agent Regulation. 42 CFR Part 73: Possession, Use, and Transfer of Select Agents and Toxins; Biennial Review; Final Rule. Fed Regist October 5, 2012;77(194). http://www.gpo.gov/fdsys/pkg/FR-2012-10-05/pdf/2012-24389.pdf Accessed August19, 2015 - PubMed
-
- Select Agent Regulation. 7 CFR Part 121, 9 CFR Part 331: Agricultural Bioterrorism Protection Act of 2002; Biennial Review and Republication of the Select Agent and Toxin List; Amendments to the Select Agent and Toxin Regulations; Final Rule. Fed Regist October 5, 2012;77(194). http://www.gpo.gov/fdsys/pkg/FR-2012-10-05/pdf/2012-24434.pdf Accessed August19, 2015
-
- Department of Health and Human Services, National Institutes of Health. NIH Guidelines for Research Involving Recombinant or Synhetic Nucleic Acid Molecules (NIH Guidelines). November 2013. http://osp.od.nih.gov/sites/default/files/NIH_Guidelines.html Accessed August19, 2015
-
- United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern (March 2012). http://www.phe.gov/s3/dualuse/Documents/us-policy-durc-032812.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

