A Change in the Ion Selectivity of Ligand-Gated Ion Channels Provides a Mechanism to Switch Behavior
- PMID: 26348462
- PMCID: PMC4562599
- DOI: 10.1371/journal.pbio.1002238
A Change in the Ion Selectivity of Ligand-Gated Ion Channels Provides a Mechanism to Switch Behavior
Abstract
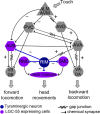
Behavioral output of neural networks depends on a delicate balance between excitatory and inhibitory synaptic connections. However, it is not known whether network formation and stability is constrained by the sign of synaptic connections between neurons within the network. Here we show that switching the sign of a synapse within a neural circuit can reverse the behavioral output. The inhibitory tyramine-gated chloride channel, LGC-55, induces head relaxation and inhibits forward locomotion during the Caenorhabditis elegans escape response. We switched the ion selectivity of an inhibitory LGC-55 anion channel to an excitatory LGC-55 cation channel. The engineered cation channel is properly trafficked in the native neural circuit and results in behavioral responses that are opposite to those produced by activation of the LGC-55 anion channel. Our findings indicate that switches in ion selectivity of ligand-gated ion channels (LGICs) do not affect network connectivity or stability and may provide an evolutionary and a synthetic mechanism to change behavior.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Ligand-gated chloride channels are receptors for biogenic amines in C. elegans.Science. 2009 Jul 3;325(5936):96-100. doi: 10.1126/science.1169243. Science. 2009. PMID: 19574391 Free PMC article.
-
A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response.Neuron. 2009 May 28;62(4):526-38. doi: 10.1016/j.neuron.2009.04.013. Neuron. 2009. PMID: 19477154 Free PMC article.
-
Activity of the C. elegans egg-laying behavior circuit is controlled by competing activation and feedback inhibition.Elife. 2016 Nov 16;5:e21126. doi: 10.7554/eLife.21126. Elife. 2016. PMID: 27849154 Free PMC article.
-
Biogenic amine receptors in parasitic nematodes: what can be learned from Caenorhabditis elegans?Mol Biochem Parasitol. 2004 Sep;137(1):1-11. doi: 10.1016/j.molbiopara.2004.05.010. Mol Biochem Parasitol. 2004. PMID: 15279946 Review.
-
Mechano-gated channels in C. elegans.J Neurogenet. 2020 Sep-Dec;34(3-4):363-368. doi: 10.1080/01677063.2020.1832091. Epub 2020 Dec 16. J Neurogenet. 2020. PMID: 33325279 Review.
Cited by
-
Drug discovery: Insights from the invertebrate Caenorhabditis elegans.Pharmacol Res Perspect. 2021 Apr;9(2):e00721. doi: 10.1002/prp2.721. Pharmacol Res Perspect. 2021. PMID: 33641258 Free PMC article. Review.
-
Co-transmission of neuropeptides and monoamines choreograph the C. elegans escape response.PLoS Genet. 2022 Mar 3;18(3):e1010091. doi: 10.1371/journal.pgen.1010091. eCollection 2022 Mar. PLoS Genet. 2022. PMID: 35239681 Free PMC article.
-
Cytoplasmic TDP-43 leads to early functional impairments without neurodegeneration in a Serotonergic Neuron-Specific C. elegans Model.bioRxiv [Preprint]. 2025 Jul 31:2025.07.30.667669. doi: 10.1101/2025.07.30.667669. bioRxiv. 2025. PMID: 40766632 Free PMC article. Preprint.
-
Understanding neural circuit function through synaptic engineering.Nat Rev Neurosci. 2024 Feb;25(2):131-139. doi: 10.1038/s41583-023-00777-8. Epub 2024 Jan 3. Nat Rev Neurosci. 2024. PMID: 38172626 Review.
-
Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms.Cells. 2022 Mar 7;11(5):914. doi: 10.3390/cells11050914. Cells. 2022. PMID: 35269536 Free PMC article. Review.
References
-
- Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol. 1998;8:149–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

