Investigating pulmonary and systemic pharmacokinetics of inhaled olodaterol in healthy volunteers using a population pharmacokinetic approach
- PMID: 26348533
- PMCID: PMC4767206
- DOI: 10.1111/bcp.12780
Investigating pulmonary and systemic pharmacokinetics of inhaled olodaterol in healthy volunteers using a population pharmacokinetic approach
Abstract
Aims: Olodaterol, a novel β2-adrenergic receptor agonist, is a long-acting, once-daily inhaled bronchodilator approved for the treatment of chronic obstructive pulmonary disease. The aim of the present study was to describe the plasma and urine pharmacokinetics of olodaterol after intravenous administration and oral inhalation in healthy volunteers by population pharmacokinetic modelling and thereby to infer its pulmonary fate.
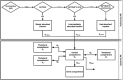
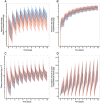
Methods: Plasma and urine data after intravenous administration (0.5-25 μg) and oral inhalation (2.5-70 μg via the Respimat® inhaler) were available from a total of 148 healthy volunteers (single and multiple dosing). A stepwise model building approach was applied, using population pharmacokinetic modelling. Systemic disposition parameters were fixed to estimates obtained from intravenous data when modelling data after inhalation.
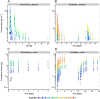
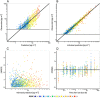
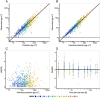
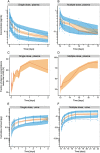
Results: A pharmacokinetic model, including three depot compartments with associated parallel first-order absorption processes (pulmonary model) on top of a four-compartment body model (systemic disposition model), was found to describe the data the best. The dose reaching the lung (pulmonary bioavailable fraction) was estimated to be 49.4% [95% confidence interval (CI) 46.1, 52.7%] of the dose released from the device. A large proportion of the pulmonary bioavailable fraction [70.1% (95% CI 66.8, 73.3%)] was absorbed with a half-life of 21.8 h (95% CI 19.7, 24.4 h).
Conclusions: The plasma and urine pharmacokinetics of olodaterol after intravenous administration and oral inhalation in healthy volunteers were adequately described. The key finding was that a high proportion of the pulmonary bioavailable fraction had an extended pulmonary residence time. This finding was not expected based on the physicochemical properties of olodaterol.
Keywords: NONMEM; inhalation; olodaterol; pharmacometrics; population pharmacokinetics; pulmonary absorption.
© 2015 The British Pharmacological Society.
Figures
Similar articles
-
Model-based evaluation of pulmonary pharmacokinetics in asthmatic and COPD patients after oral olodaterol inhalation.Br J Clin Pharmacol. 2016 Sep;82(3):739-53. doi: 10.1111/bcp.12999. Epub 2016 Jun 23. Br J Clin Pharmacol. 2016. PMID: 27145733 Free PMC article. Clinical Trial.
-
A randomised, placebo-controlled, Phase II, dose-ranging trial of once-daily treatment with olodaterol, a novel long-acting β2-agonist, for 4 weeks in patients with chronic obstructive pulmonary disease.Respir Med. 2015 May;109(5):596-605. doi: 10.1016/j.rmed.2015.02.012. Epub 2015 Mar 3. Respir Med. 2015. PMID: 25829298 Clinical Trial.
-
Efficacy and safety of the long-acting β2-agonist olodaterol over 4 weeks in Japanese patients with chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2015 Aug 20;10:1673-83. doi: 10.2147/COPD.S86002. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26316741 Free PMC article. Clinical Trial.
-
Combined bronchodilators (tiotropium plus olodaterol) for patients with chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2015 Oct 30;10:2347-56. doi: 10.2147/COPD.S88246. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26586940 Free PMC article. Review.
-
Olodaterol for the treatment of chronic obstructive pulmonary disease: a narrative review.Expert Opin Pharmacother. 2018 Oct;19(14):1603-1611. doi: 10.1080/14656566.2018.1518431. Epub 2018 Oct 12. Expert Opin Pharmacother. 2018. PMID: 30311516 Review.
Cited by
-
Functionalization of iron oxide nanoparticles with clove extract to induce apoptosis in MCF-7 breast cancer cells.3 Biotech. 2020 Feb;10(2):82. doi: 10.1007/s13205-020-2088-7. Epub 2020 Feb 1. 3 Biotech. 2020. PMID: 32099733 Free PMC article.
-
Inhalable Nano Formulation of Cabazitaxel: A Comparative Study with Intravenous Route.Macromol Biosci. 2025 May;25(5):e2400567. doi: 10.1002/mabi.202400567. Epub 2025 Jan 30. Macromol Biosci. 2025. PMID: 39888152 Free PMC article.
-
Model-based evaluation of pulmonary pharmacokinetics in asthmatic and COPD patients after oral olodaterol inhalation.Br J Clin Pharmacol. 2016 Sep;82(3):739-53. doi: 10.1111/bcp.12999. Epub 2016 Jun 23. Br J Clin Pharmacol. 2016. PMID: 27145733 Free PMC article. Clinical Trial.
-
Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes.Can Respir J. 2018 Jun 19;2018:2732017. doi: 10.1155/2018/2732017. eCollection 2018. Can Respir J. 2018. PMID: 30018677 Free PMC article. Review.
-
Pharmacokinetics of the Inhaled Selective Glucocorticoid Receptor Modulator AZD5423 Following Inhalation Using Different Devices.AAPS J. 2017 May;19(3):865-874. doi: 10.1208/s12248-016-0042-8. Epub 2017 Mar 9. AAPS J. 2017. PMID: 28281196 Clinical Trial.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2015. Available from: http://www.goldcopd.org/ - PubMed
-
- Gibb A, Yang LP. Olodaterol: first global approval. Drugs 2013; 73: 1841–6. - PubMed
-
- Olodaterol (Striverdi Respimat) for COPD . Med Lett Drugs Ther 2015; 57: 1–3. - PubMed
-
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta‐analysis. Arch Intern Med 1999; 159: 941–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

