Asymmetric Redox-Annulation of Cyclic Amines
- PMID: 26348653
- PMCID: PMC4599696
- DOI: 10.1021/acs.joc.5b01384
Asymmetric Redox-Annulation of Cyclic Amines
Abstract
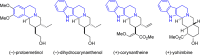
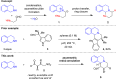
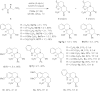
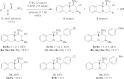
Cyclic amines such as 1,2,3,4-tetrahydroisoquinoline undergo regiodivergent annulation reactions with 4-nitrobutyraldehydes. These redox-neutral transformations enable the asymmetric synthesis of highly substituted polycyclic ring systems in just two steps from commercial materials. The utility of this process is illustrated in a rapid synthesis of (-)-protoemetinol. Computational studies provide mechanistic insights and implicate the elimination of acetic acid from an ammonium nitronate intermediate as the rate-determining step.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
-
For selected recent syntheses of the compounds shown in Scheme 1, see:
- Ihara M.; Yasui K.; Taniguchi N.; Fukumoto K. J. Chem. Soc., Perkin Trans. 1 1990, 1469. 10.1039/p19900001469. - DOI
- Itoh T.; Yokoya M.; Miyauchi K.; Nagata K.; Ohsawa A. Org. Lett. 2006, 8, 1533. 10.1021/ol0530575. - DOI - PubMed
- Mergott D. J.; Zuend S. J.; Jacobsen E. N. Org. Lett. 2008, 10, 745. 10.1021/ol702781q. - DOI - PubMed
- Chang J.-K.; Chang B.-R.; Chuang Y.-H.; Chang N.-C. Tetrahedron 2008, 64, 9685. 10.1016/j.tet.2008.07.110. - DOI
- Nuhant P.; Raikar S. B.; Wypych J.-C.; Delpech B.; Marazano C. J. Org. Chem. 2009, 74, 9413. 10.1021/jo9019545. - DOI - PubMed
- English B. J.; Williams R. M. J. Org. Chem. 2010, 75, 7869. 10.1021/jo101775n. - DOI - PMC - PubMed
- Sun X.; Ma D. Chem. - Asian J. 2011, 6, 2158. 10.1002/asia.201100219. - DOI - PubMed
- Wanner M. J.; Claveau E.; van Maarseveen J. H.; Hiemstra H. Chem. - Eur. J. 2011, 17, 13680. 10.1002/chem.201103150. - DOI - PubMed
- Zhang W.; Bah J.; Wohlfarth A.; Franzén J. Chem. - Eur. J. 2011, 17, 13814. 10.1002/chem.201102012. - DOI - PubMed
- Herle B.; Wanner M. J.; van Maarseveen J. H.; Hiemstra H. J. Org. Chem. 2011, 76, 8907. 10.1021/jo201657n. - DOI - PubMed
- Palframan M. J.; Parsons A. F.; Johnson P. Tetrahedron Lett. 2011, 52, 1154. 10.1016/j.tetlet.2011.01.007. - DOI
- Lin S.; Deiana L.; Tseggai A.; Córdova A. Eur. J. Org. Chem. 2012, 2012, 398. 10.1002/ejoc.201101296. - DOI
- Lebold T. P.; Wood J. L.; Deitch J.; Lodewyk M. W.; Tantillo D. J.; Sarpong R. Nat. Chem. 2012, 5, 126. 10.1038/nchem.1528. - DOI - PMC - PubMed
- Moon H.; An H.; Sim J.; Kim K.; Paek S.-M.; Suh Y.-G. Tetrahedron Lett. 2015, 56, 608. 10.1016/j.tetlet.2014.12.030. - DOI
-
-
-
Examples from our laboratory:
- Zhang C.; De C. K.; Mal R.; Seidel D. J. Am. Chem. Soc. 2008, 130, 416. 10.1021/ja077473r. - DOI - PubMed
- Zhang C.; Das D.; Seidel D. Chem. Sci. 2011, 2, 233. 10.1039/C0SC00432D. - DOI
- Ma L.; Chen W.; Seidel D. J. Am. Chem. Soc. 2012, 134, 15305. 10.1021/ja308009g. - DOI - PubMed
- Das D.; Sun A. X.; Seidel D. Angew. Chem., Int. Ed. 2013, 52, 3765. 10.1002/anie.201300021. - DOI - PMC - PubMed
- Dieckmann A.; Richers M. T.; Platonova A. Y.; Zhang C.; Seidel D.; Houk K. N. J. Org. Chem. 2013, 78, 4132. 10.1021/jo400483h. - DOI - PMC - PubMed
- Chen W.; Wilde R. G.; Seidel D. Org. Lett. 2014, 16, 730. 10.1021/ol403431u. - DOI - PMC - PubMed
- Chen W.; Kang Y.; Wilde R. G.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5179. 10.1002/anie.201311165. - DOI - PMC - PubMed
- Richers M. T.; Breugst M.; Platonova A. Y.; Ullrich A.; Dieckmann A.; Houk K. N.; Seidel D. J. Am. Chem. Soc. 2014, 136, 6123. 10.1021/ja501988b. - DOI - PMC - PubMed
- Chen W.; Seidel D. Org. Lett. 2014, 16, 3158. 10.1021/ol501365j. - DOI - PMC - PubMed
- Jarvis C. L.; Richers M. T.; Breugst M.; Houk K. N.; Seidel D. Org. Lett. 2014, 16, 3556. 10.1021/ol501509b. - DOI - PMC - PubMed
-
-
-
Selected recent reviews on amine α-functionalization, including redox-neutral approaches:
- Campos K. R. Chem. Soc. Rev. 2007, 36, 1069. 10.1039/b607547a. - DOI - PubMed
- Jazzar R.; Hitce J.; Renaudat A.; Sofack-Kreutzer J.; Baudoin O. Chem. - Eur. J. 2010, 16, 2654. 10.1002/chem.200902374. - DOI - PubMed
- Mitchell E. A.; Peschiulli A.; Lefevre N.; Meerpoel L.; Maes B. U. W. Chem. - Eur. J. 2012, 18, 10092. 10.1002/chem.201201539. - DOI - PubMed
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322. 10.1021/cr300503r. - DOI - PMC - PubMed
- Peng B.; Maulide N. Chem. - Eur. J. 2013, 19, 13274. 10.1002/chem.201301522. - DOI - PubMed
- Wang L.; Xiao J. Adv. Synth. Catal. 2014, 356, 1137. 10.1002/adsc.201301153. - DOI
- Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74. 10.1002/anie.201304268. - DOI - PubMed
- Vo C.-V. T.; Bode J. W. J. Org. Chem. 2014, 79, 2809. 10.1021/jo5001252. - DOI - PubMed
- Haibach M. C.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5010. 10.1002/anie.201306489. - DOI - PubMed
- Seidel D. Acc. Chem. Res. 2015, 48, 317. 10.1021/ar5003768. - DOI - PMC - PubMed
-
-
-
Additional reviews covering various aspects of redox-neutral chemistry
- Burns N. Z.; Baran P. S.; Hoffmann R. W. Angew. Chem., Int. Ed. 2009, 48, 2854. 10.1002/anie.200806086. - DOI - PubMed
- Mahatthananchai J.; Bode J. W. Acc. Chem. Res. 2014, 47, 696. 10.1021/ar400239v. - DOI - PubMed
- Ketcham J. M.; Shin I.; Montgomery T. P.; Krische M. J. Angew. Chem., Int. Ed. 2014, 53, 9142. 10.1002/anie.201403873. - DOI - PMC - PubMed
- Huang H.; Ji X.; Wu W.; Jiang H. Chem. Soc. Rev. 2015, 44, 1155. 10.1039/C4CS00288A. - DOI - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

