Analyzing and Modeling the Kinetics of Amyloid Beta Pores Associated with Alzheimer's Disease Pathology
- PMID: 26348728
- PMCID: PMC4562663
- DOI: 10.1371/journal.pone.0137357
Analyzing and Modeling the Kinetics of Amyloid Beta Pores Associated with Alzheimer's Disease Pathology
Abstract
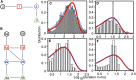
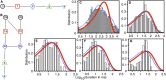
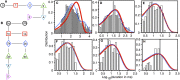
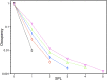
Amyloid beta (Aβ) oligomers associated with Alzheimer's disease (AD) form Ca2+-permeable plasma membrane pores, leading to a disruption of the otherwise well-controlled intracellular calcium (Ca2+) homeostasis. The resultant up-regulation of intracellular Ca2+ concentration has detrimental implications for memory formation and cell survival. The gating kinetics and Ca2+ permeability of Aβ pores are not well understood. We have used computational modeling in conjunction with the ability of optical patch-clamping for massively parallel imaging of Ca2+ flux through thousands of pores in the cell membrane of Xenopus oocytes to elucidate the kinetic properties of Aβ pores. The fluorescence time-series data from individual pores were idealized and used to develop data-driven Markov chain models for the kinetics of the Aβ pore at different stages of its evolution. Our study provides the first demonstration of developing Markov chain models for ion channel gating that are driven by optical-patch clamp data with the advantage of experiments being performed under close to physiological conditions. Towards the end, we demonstrate the up-regulation of gating of various Ca2+ release channels due to Aβ pores and show that the extent and spatial range of such up-regulation increases as Aβ pores with low open probability and Ca2+ permeability transition into those with high open probability and Ca2+ permeability.
Conflict of interest statement
Figures
References
-
- Hardy J. A. and Higgins G. A. (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science, 256:184–185. - PubMed
-
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., et al. (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature, 359:322–325. - PubMed
-
- Demuro A., Mina E., Kayed R., Milton S. C., Parker I., and Glabe C. G. (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem., 280:17294–17300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

