Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era
- PMID: 26348734
- PMCID: PMC4948649
- DOI: 10.1001/jamainternmed.2015.4114
Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era
Abstract
Importance: Clostridium difficile is a major cause of health care-associated infection, but disagreement between diagnostic tests is an ongoing barrier to clinical decision making and public health reporting. Molecular tests are increasingly used to diagnose C difficile infection (CDI), but many molecular test-positive patients lack toxins that historically defined disease, making it unclear if they need treatment.
Objective: To determine the natural history and need for treatment of patients who are toxin immunoassay negative and polymerase chain reaction (PCR) positive (Tox-/PCR+) for CDI.
Design, setting, and participants: Prospective observational cohort study at a single academic medical center among 1416 hospitalized adults tested for C difficile toxins 72 hours or longer after admission between December 1, 2010, and October 20, 2012. The analysis was conducted in stages with revisions from April 27, 2013, to January 13, 2015.
Main outcomes and measures: Patients undergoing C difficile testing were grouped by US Food and Drug Administration-approved toxin and PCR tests as Tox+/PCR+, Tox-/PCR+, or Tox-/PCR-. Toxin results were reported clinically. Polymerase chain reaction results were not reported. The main study outcomes were duration of diarrhea during up to 14 days of treatment, rate of CDI-related complications (ie, colectomy, megacolon, or intensive care unit care) and CDI-related death within 30 days.
Results: Twenty-one percent (293 of 1416) of hospitalized adults tested for C difficile were positive by PCR, but 44.7% (131 of 293) had toxins detected by the clinical toxin test. At baseline, Tox-/PCR+ patients had lower C difficile bacterial load and less antibiotic exposure, fecal inflammation, and diarrhea than Tox+/PCR+ patients (P < .001 for all). The median duration of diarrhea was shorter in Tox-/PCR+ patients (2 days; interquartile range, 1-4 days) than in Tox+/PCR+ patients (3 days; interquartile range, 1-6 days) (P = .003) and was similar to that in Tox-/PCR- patients (2 days; interquartile range, 1-3 days), despite minimal empirical treatment of Tox-/PCR+ patients. No CDI-related complications occurred in Tox-/PCR+ patients vs 10 complications in Tox+/PCR+ patients (0% vs 7.6%, P < .001). One Tox-/PCR+ patient had recurrent CDI as a contributing factor to death within 30 days vs 11 CDI-related deaths in Tox+/PCR+ patients (0.6% vs 8.4%, P = .001).
Conclusions and relevance: Among hospitalized adults with suspected CDI, virtually all CDI-related complications and deaths occurred in patients with positive toxin immunoassay test results. Patients with a positive molecular test result and a negative toxin immunoassay test result had outcomes that were comparable to patients without C difficile by either method. Exclusive reliance on molecular tests for CDI diagnosis without tests for toxins or host response is likely to result in overdiagnosis, overtreatment, and increased health care costs.
Figures
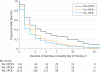
Comment in
-
Diagnosis of Clostridium difficile Infection: Treat the Patient, Not the Test.JAMA Intern Med. 2015 Nov;175(11):1801-2. doi: 10.1001/jamainternmed.2015.4607. JAMA Intern Med. 2015. PMID: 26348248 No abstract available.
-
Toxin Immunoassays and Clostridium difficile Infection.JAMA Intern Med. 2016 Mar;176(3):412-3. doi: 10.1001/jamainternmed.2015.8522. JAMA Intern Med. 2016. PMID: 26954047 No abstract available.
-
Toxin Immunoassays and Clostridium difficile Infection.JAMA Intern Med. 2016 Mar;176(3):413. doi: 10.1001/jamainternmed.2015.8525. JAMA Intern Med. 2016. PMID: 26954049 No abstract available.
-
Toxin Immunoassays and Clostridium difficile Infection.JAMA Intern Med. 2016 Mar;176(3):413-4. doi: 10.1001/jamainternmed.2015.8536. JAMA Intern Med. 2016. PMID: 26954050 No abstract available.
-
Toxin Immunoassays and Clostridium difficile Infection-Reply.JAMA Intern Med. 2016 Mar;176(3):414-5. doi: 10.1001/jamainternmed.2015.8539. JAMA Intern Med. 2016. PMID: 26954051 No abstract available.
-
Welche Aussagekraft haben Toxin- und PCR-Tests?Z Gastroenterol. 2016 Mar;54(3):205-6. Z Gastroenterol. 2016. PMID: 27500295 German. No abstract available.
Similar articles
-
Clinical heterogeneity of patients with stool samples testing PCR+/Tox- from a two-step Clostridium difficile diagnostic algorithm.Eur J Clin Microbiol Infect Dis. 2018 Dec;37(12):2355-2359. doi: 10.1007/s10096-018-3383-7. Epub 2018 Sep 20. Eur J Clin Microbiol Infect Dis. 2018. PMID: 30238342
-
Impact of simultaneous glutamate dehydrogenase and toxin A/B rapid immunoassay on Clostridium difficile diagnosis and treatment in hospitalized patients with antibiotic-associated diarrhea in a university hospital of Brazil.J Gastroenterol Hepatol. 2018 Feb;33(2):393-396. doi: 10.1111/jgh.13901. J Gastroenterol Hepatol. 2018. PMID: 28730697
-
Does empirical Clostridium difficile infection (CDI) therapy result in false-negative CDI diagnostic test results?Clin Infect Dis. 2013 Aug;57(4):494-500. doi: 10.1093/cid/cit286. Epub 2013 May 3. Clin Infect Dis. 2013. PMID: 23645849
-
Diagnosis and management of Clostridium difficile infection.Clin Gastroenterol Hepatol. 2013 Oct;11(10):1216-23; quiz e73. doi: 10.1016/j.cgh.2013.03.016. Epub 2013 Mar 28. Clin Gastroenterol Hepatol. 2013. PMID: 23542332 Review.
-
Rapid detection of Clostridium difficile toxins and laboratory diagnosis of Clostridium difficile infections.Infection. 2017 Jun;45(3):255-262. doi: 10.1007/s15010-016-0940-9. Epub 2016 Sep 6. Infection. 2017. PMID: 27601055 Review.
Cited by
-
Trends in the incidence of Clostridioides difficile infection in adults and the elderly insured by Medicaid compared to commercial insurance or Medicare only.Infect Control Hosp Epidemiol. 2023 Jul;44(7):1076-1084. doi: 10.1017/ice.2022.208. Epub 2022 Sep 9. Infect Control Hosp Epidemiol. 2023. PMID: 36082779 Free PMC article.
-
Atypical presentation of Clostridioides difficile pseudomembranous colitis with laboratory rejection of stool specimen.BMJ Case Rep. 2019 Nov 26;12(11):e230629. doi: 10.1136/bcr-2019-230629. BMJ Case Rep. 2019. PMID: 31776146 Free PMC article.
-
Clostridioides difficile infection in cancer patients receiving immune checkpoint inhibitors.Ann Gastroenterol. 2022 Jul-Aug;35(4):393-399. doi: 10.20524/aog.2022.0722. Epub 2022 Jun 2. Ann Gastroenterol. 2022. PMID: 35784625 Free PMC article.
-
Reducing rates of Clostridium difficile infection by switching to a stand-alone NAAT with clear sampling criteria.Antimicrob Resist Infect Control. 2018 Mar 12;7:40. doi: 10.1186/s13756-018-0332-2. eCollection 2018. Antimicrob Resist Infect Control. 2018. PMID: 29564088 Free PMC article.
-
Guidelines for Clostridium difficile infection in adults.Prz Gastroenterol. 2020;15(1):1-21. doi: 10.5114/pg.2020.93629. Epub 2020 Mar 19. Prz Gastroenterol. 2020. PMID: 32215122 Free PMC article.
References
-
- Lucado J, Gould C, Elixhauser A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Rockville, MD: Agency for Health Care Policy and Research; 2006-2012. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs - PubMed
-
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7(7):526–536. - PubMed
-
- McDonald LC, Lessa F, Sievert D, et al. Centers for Disease Control and Prevention (CDC). Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61(9):157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

