Role of N-glycosylation in renal betaine transport
- PMID: 26348906
- PMCID: PMC5070602
- DOI: 10.1042/BJ20131031
Role of N-glycosylation in renal betaine transport
Abstract
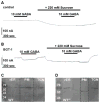
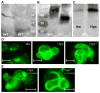
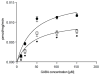
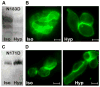
The osmolyte and folding chaperone betaine is transported by the renal Na(+)-coupled GABA (γ-aminobutyric acid) symporter BGT-1 (betaine/GABA transporter 1), a member of the SLC6 (solute carrier 6) family. Under hypertonic conditions, the transcription, translation and plasma membrane (PM) insertion of BGT-1 in kidney cells are significantly increased, resulting in elevated betaine and GABA transport. Re-establishing isotonicity involves PM depletion of BGT-1. The molecular mechanism of the regulated PM insertion of BGT-1 during changes in osmotic stress is unknown. In the present study, we reveal a link between regulated PM insertion and N-glycosylation. Based on homology modelling, we identified two sites (Asn(171) and Asn(183)) in the extracellular loop 2 (EL2) of BGT-1, which were investigated with respect to trafficking, insertion and transport by immunogold-labelling, electron microscopy (EM), mutagenesis and two-electrode voltage clamp measurements in Xenopus laevis oocytes and uptake of radiolabelled substrate into MDCK (Madin-Darby canine kidney) and HEK293 (human embryonic kidney) cells. Trafficking and PM insertion of BGT-1 was clearly promoted by N-glycosylation in both oocytes and MDCK cells. Moreover, association with N-glycans at Asn(171) and Asn(183) contributed equally to protein activity and substrate affinity. Substitution of Asn(171) and Asn(183) by aspartate individually caused no loss of BGT-1 activity, whereas the double mutant was inactive, suggesting that N-glycosylation of at least one of the sites is required for function. Substitution by alanine or valine at either site caused a dramatic loss in transport activity. Furthermore, in MDCK cells PM insertion of N183D was no longer regulated by osmotic stress, highlighting the impact of N-glycosylation in regulation of this SLC6 transporter.
Keywords: kidney; neurotransmitter:sodium symporter (NSS)/solute carrier 6 (SLC6) family; osmotic stress response; regulation; subcellular distribution; transport; γ-aminobutyric acid (GABA).
© 2015 Authors; published by Portland Press Limited.
Figures


Similar articles
-
Functional characterization of the Betaine/gamma-aminobutyric acid transporter BGT-1 expressed in Xenopus oocytes.J Biol Chem. 1999 Jun 11;274(24):16709-16. doi: 10.1074/jbc.274.24.16709. J Biol Chem. 1999. PMID: 10358010
-
Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity.J Biol Chem. 1992 Jan 5;267(1):649-52. J Biol Chem. 1992. PMID: 1370453
-
Subcellular redistribution of the renal betaine transporter during hypertonic stress.Am J Physiol Cell Physiol. 2003 Nov;285(5):C1091-100. doi: 10.1152/ajpcell.00021.2003. Epub 2003 Jul 2. Am J Physiol Cell Physiol. 2003. PMID: 12839828
-
Osmotic regulation of renal betaine transport: transcription and beyond.Pflugers Arch. 2004 Dec;449(3):227-34. doi: 10.1007/s00424-004-1338-6. Pflugers Arch. 2004. PMID: 15452713 Review.
-
Regulation of renal cell organic osmolyte transport by tonicity.Am J Physiol. 1993 Dec;265(6 Pt 1):C1449-55. doi: 10.1152/ajpcell.1993.265.6.C1449. Am J Physiol. 1993. PMID: 8279508 Review.
References
-
- Burg MB. Molecular basis of osmotic regulation. Am J Physiol Renal Physiol. 1995;268:F983–996. - PubMed
-
- Kwon HM, Handler JS. Cell volume regulated transporters of compatible osmolytes. Curr Opin Cell Biol. 1995;7:465–471. - PubMed
-
- Beck FX, Burger-Kentischer A, Muller E. Cellular response to osmotic stress in the renal medulla. Pflügers Arch. 1998;436:814–827. - PubMed
-
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

