GSK3-mediated raptor phosphorylation supports amino-acid-dependent mTORC1-directed signalling
- PMID: 26348909
- PMCID: PMC4652938
- DOI: 10.1042/BJ20150404
GSK3-mediated raptor phosphorylation supports amino-acid-dependent mTORC1-directed signalling
Abstract
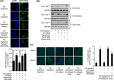
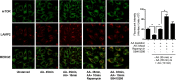
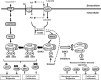
The mammalian or mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) is a ubiquitously expressed multimeric protein kinase complex that integrates nutrient and growth factor signals for the co-ordinated regulation of cellular metabolism and cell growth. Herein, we demonstrate that suppressing the cellular activity of glycogen synthase kinase-3 (GSK3), by use of pharmacological inhibitors or shRNA-mediated gene silencing, results in substantial reduction in amino acid (AA)-regulated mTORC1-directed signalling, as assessed by phosphorylation of multiple downstream mTORC1 targets. We show that GSK3 regulates mTORC1 activity through its ability to phosphorylate the mTOR-associated scaffold protein raptor (regulatory-associated protein of mTOR) on Ser(859). We further demonstrate that either GSK3 inhibition or expression of a S859A mutated raptor leads to reduced interaction between mTOR and raptor and under these circumstances, irrespective of AA availability, there is a consequential loss in phosphorylation of mTOR substrates, such as p70S6K1 (ribosomal S6 kinase 1) and uncoordinated-51-like kinase (ULK1), which results in increased autophagic flux and reduced cellular proliferation.
Keywords: L-type (leucine) amino acid transporter 1 (LAT1); amino acid; autophagy; growth; insulin; leucine; p70S6K1; proliferation; sodium-coupled neutral amino acid transporter 2 (SNAT2); transcription factor EB (TFEB); uncoordinated-51-like kinase (ULK1).
© 2015 Authors; published by Portland Press Limited.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

