Widespread Expression of Erythropoietin Receptor in Brain and Its Induction by Injury
- PMID: 26349059
- PMCID: PMC4818269
- DOI: 10.2119/molmed.2015.00192
Widespread Expression of Erythropoietin Receptor in Brain and Its Induction by Injury
Abstract
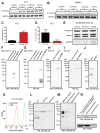
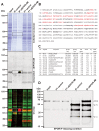
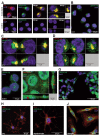
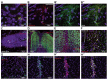
Erythropoietin (EPO) exerts potent neuroprotective, neuroregenerative and procognitive functions. However, unequivocal demonstration of erythropoietin receptor (EPOR) expression in brain cells has remained difficult since previously available anti-EPOR antibodies (EPOR-AB) were unspecific. We report here a new, highly specific, polyclonal rabbit EPOR-AB directed against different epitopes in the cytoplasmic tail of human and murine EPOR and its characterization by mass spectrometric analysis of immuno-precipitated endogenous EPOR, Western blotting, immunostaining and flow cytometry. Among others, we applied genetic strategies including overexpression, Lentivirus-mediated conditional knockout of EpoR and tagged proteins, both on cultured cells and tissue sections, as well as intracortical implantation of EPOR-transduced cells to verify specificity. We show examples of EPOR expression in neurons, oligodendroglia, astrocytes and microglia. Employing this new EPOR-AB with double-labeling strategies, we demonstrate membrane expression of EPOR as well as its localization in intracellular compartments such as the Golgi apparatus. Moreover, we show injury-induced expression of EPOR. In mice, a stereotactically applied stab wound to the motor cortex leads to distinct EpoR expression by reactive GFAP-expressing cells in the lesion vicinity. In a patient suffering from epilepsy, neurons and oligodendrocytes of the hippocampus strongly express EPOR. To conclude, this new analytical tool will allow neuroscientists to pinpoint EPOR expression in cells of the nervous system and to better understand its role in healthy conditions, including brain development, as well as under pathological circumstances, such as upregulation upon distress and injury.
Figures
References
-
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–94. - PubMed
-
- Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205. - PubMed
-
- Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. - PubMed
-
- Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24:573–94. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

