Synthesis of aza and carbocyclic β-carbolines for the treatment of alcohol abuse. Regiospecific solution to the problem of 3,6-disubstituted β- and aza-β-carboline specificity
- PMID: 26349488
- PMCID: PMC4704088
- DOI: 10.1039/c5ob01572c
Synthesis of aza and carbocyclic β-carbolines for the treatment of alcohol abuse. Regiospecific solution to the problem of 3,6-disubstituted β- and aza-β-carboline specificity
Abstract
A novel two step protocol was developed to gain regiospecific access to 3-substituted β- and aza-β-carbolines, 3-PBC (1), 3-ISOPBC (2), βCCt (3), 6-aza-3-PBC (4) and 6-aza-3-ISOPBC (5). These β-carbolines (1-3) are potential clinical agents to reduce alcohol self-administration, especially 3-ISOPBC·HCl (2·HCl) which appears to be a potent anti-alcohol agent active against binge drinking in a rat model of maternally deprived (MD) rats. The method consists of two consecutive palladium-catalyzed reactions: a Buchwald-Hartwig amination followed by an intramolecular Heck-type cyclization in high yield.
Figures
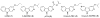

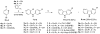

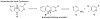


Similar articles
-
Development of a two-step route to 3-PBC and βCCt, two agents active against alcohol self-administration in rodent and primate models.J Org Chem. 2011 Jun 3;76(11):4721-7. doi: 10.1021/jo200425m. Epub 2011 May 4. J Org Chem. 2011. PMID: 21495660 Free PMC article.
-
Domino Heck-aza-Michael reactions: efficient access to 1-substituted tetrahydro-beta-carbolines.J Org Chem. 2010 Mar 5;75(5):1787-90. doi: 10.1021/jo902652h. J Org Chem. 2010. PMID: 20131765
-
Design, synthesis, and subtype selectivity of 3,6-disubstituted β-carbolines at Bz/GABA(A)ergic receptors. SAR and studies directed toward agents for treatment of alcohol abuse.Bioorg Med Chem. 2010 Nov 1;18(21):7548-64. doi: 10.1016/j.bmc.2010.08.049. Epub 2010 Sep 29. Bioorg Med Chem. 2010. PMID: 20888240 Free PMC article.
-
Synthesis of aza-heterocycles from oximes by amino-Heck reaction.Chem Rec. 2002;2(4):268-77. doi: 10.1002/tcr.10030. Chem Rec. 2002. PMID: 12203909 Review.
-
Structure activity relationship in β-carboline derived anti-malarial agents.Eur J Med Chem. 2021 Oct 5;221:113536. doi: 10.1016/j.ejmech.2021.113536. Epub 2021 May 13. Eur J Med Chem. 2021. PMID: 34058709 Review.
Cited by
-
Looking back at a life in sleep research-and some thoughts for the future.Sleep Adv. 2022 Sep 12;3(1):zpac031. doi: 10.1093/sleepadvances/zpac031. eCollection 2022. Sleep Adv. 2022. PMID: 37193401 Free PMC article.
-
Effects of the benzodiazepine GABAA α1-preferring antagonist 3-isopropoxy-β-carboline hydrochloride (3-ISOPBC) on alcohol seeking and self-administration in baboons.Drug Alcohol Depend. 2017 Jan 1;170:25-31. doi: 10.1016/j.drugalcdep.2016.10.036. Epub 2016 Nov 4. Drug Alcohol Depend. 2017. PMID: 27865151 Free PMC article.
References
-
- Cao R, Peng W, Wang Z, Xu A. Curr Med Chem. 2007;14:479–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

