Meropenem-RPX7009 Concentrations in Plasma, Epithelial Lining Fluid, and Alveolar Macrophages of Healthy Adult Subjects
- PMID: 26349830
- PMCID: PMC4649232
- DOI: 10.1128/AAC.01713-15
Meropenem-RPX7009 Concentrations in Plasma, Epithelial Lining Fluid, and Alveolar Macrophages of Healthy Adult Subjects
Abstract
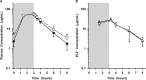
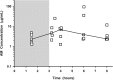
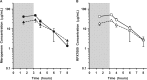
The steady-state concentrations of meropenem and the β-lactamase inhibitor RPX7009 in plasma, epithelial lining fluid (ELF), and alveolar macrophage (AM) concentrations were obtained in 25 healthy, nonsmoking adult subjects. Subjects received a fixed combination of meropenem (2 g) and RPX7009 (2 g) administered every 8 h, as a 3-h intravenous infusion, for a total of three doses. A bronchoscopy and bronchoalveolar lavage were performed once in each subject at 1.5, 3.25, 4, 6, or 8 h after the start of the last infusion. Meropenem and RPX7009 achieved a similar time course and magnitude of concentrations in plasma and ELF. The mean pharmacokinetic parameters ± the standard deviations of meropenem and RPX7009 determined from serial plasma concentrations were as follows: Cmax = 58.2 ± 10.8 and 59.0 ± 8.4 μg/ml, Vss = 16.3 ± 2.6 and 17.6 ± 2.6 liters; CL = 11.1 ± 2.1 and 10.1 ± 1.9 liters/h, and t1/2 = 1.03 ± 0.15 and 1.27 ± 0.21 h, respectively. The intrapulmonary penetrations of meropenem and RPX7009 were ca. 63 and 53%, respectively, based on the area under the concentration-time curve from 0 to 8 h (AUC0-8) values of ELF and total plasma concentrations. When unbound plasma concentrations were considered, ELF penetrations were 65 and 79% for meropenem and RPX7009, respectively. Meropenem concentrations in AMs were below the quantitative limit of detection, whereas median concentrations of RPX7009 in AMs ranged from 2.35 to 6.94 μg/ml. The results from the present study lend support to exploring a fixed combination of meropenem (2 g) and RPX7009 (2 g) for the treatment of lower respiratory tract infections caused by meropenem-resistant Gram-negative pathogens susceptible to the combination of meropenem-RPX7009.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Livermore DM. 2011. British Society for Antimicrobial Chemotherapy Working Party on The Urgent Need: Regenerating Antibacterial Drug Discovery and Development. Discovery research: the scientific challenge of finding new antibiotics. J Antimicrob Chemother 66:1941–1944. - PubMed
-
- U.S. Department of Health and Human Services/Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

