Perivascular tissue inhibits rho-kinase-dependent smooth muscle Ca(2+) sensitivity and endothelium-dependent H2 S signalling in rat coronary arteries
- PMID: 26350036
- PMCID: PMC4626546
- DOI: 10.1113/JP271006
Perivascular tissue inhibits rho-kinase-dependent smooth muscle Ca(2+) sensitivity and endothelium-dependent H2 S signalling in rat coronary arteries
Abstract
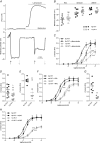
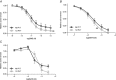
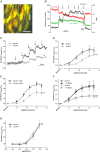
Interactions between perivascular tissue (PVT) and the vascular wall modify artery tone and contribute to local blood flow regulation. Using isometric myography, fluorescence microscopy, membrane potential recordings and phosphospecific immunoblotting, we investigated the cellular mechanisms by which PVT affects constriction and relaxation of rat coronary septal arteries. PVT inhibited vasoconstriction to thromboxane, serotonin and α1 -adrenergic stimulation but not to depolarization with elevated extracellular [K(+) ]. When PVT was wrapped around isolated arteries or placed at the bottom of the myograph chamber, a smaller yet significant inhibition of vasoconstriction was observed. Resting membrane potential, depolarization to serotonin or thromboxane stimulation, and resting and serotonin-stimulated vascular smooth muscle [Ca(2+) ]-levels were unaffected by PVT. Serotonin-induced vasoconstriction was almost abolished by rho-kinase inhibitor Y-27632 and modestly reduced by protein kinase C inhibitor bisindolylmaleimide X. PVT reduced phosphorylation of myosin phosphatase targeting subunit (MYPT) at Thr850 by ∼40% in serotonin-stimulated arteries but had no effect on MYPT-phosphorylation in arteries depolarized with elevated extracellular [K(+) ]. The net anti-contractile effect of PVT was accentuated after endothelial denudation. PVT also impaired vasorelaxation and endothelial Ca(2+) responses to cholinergic stimulation. Methacholine-induced vasorelaxation was mediated by NO and H2 S, and particularly the H2 S-dependent (dl-propargylglycine- and XE991-sensitive) component was attenuated by PVT. Vasorelaxation to NO- and H2 S-donors was maintained in arteries with PVT. In conclusion, cardiomyocyte-rich PVT surrounding coronary arteries releases diffusible factors that reduce rho-kinase-dependent smooth muscle Ca(2+) sensitivity and endothelial Ca(2+) responses. These mechanisms inhibit agonist-induced vasoconstriction and endothelium-dependent vasorelaxation and suggest new signalling pathways for metabolic regulation of blood flow.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures





References
-
- Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, Pemberton PW, Ammori B, Malik RA, Soran H & Heagerty AM (2013). Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity.J Am Coll Cardiol 62, 128–135. - PMC - PubMed
-
- Bell RM & Yellon DM (2012). Conditioning the whole heart – not just the cardiomyocyte. J Mol Cell Cardiol 53, 24–32. - PubMed
-
- Bhattacharya I, Dragert K, Albert V, Contassot E, Damjanovic M, Hagiwara A, Zimmerli L, Humar R, Hall MN, Battegay EJ & Haas E (2013). Rictor in perivascular adipose tissue controls vascular function by regulating inflammatory molecule expression. Arterioscler Thromb Vasc Biol 33, 2105–2111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

