G-quadruplexes and their regulatory roles in biology
- PMID: 26350216
- PMCID: PMC4605312
- DOI: 10.1093/nar/gkv862
G-quadruplexes and their regulatory roles in biology
Abstract
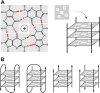
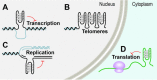
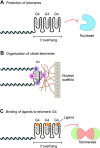
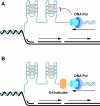
'If G-quadruplexes form so readily in vitro, Nature will have found a way of using them in vivo' (Statement by Aaron Klug over 30 years ago).During the last decade, four-stranded helical structures called G-quadruplex (or G4) have emerged from being a structural curiosity observed in vitro, to being recognized as a possible nucleic acid based mechanism for regulating multiple biological processes in vivo. The sequencing of many genomes has revealed that they are rich in sequence motifs that have the potential to form G-quadruplexes and that their location is non-random, correlating with functionally important genomic regions. In this short review, we summarize recent evidence for the in vivo presence and function of DNA and RNA G-quadruplexes in various cellular pathways including DNA replication, gene expression and telomere maintenance. We also highlight remaining open questions that will have to be addressed in the future.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Sundquist W.I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989;342:825–829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

