Novel Approaches Reveal that Toxoplasma gondii Bradyzoites within Tissue Cysts Are Dynamic and Replicating Entities In Vivo
- PMID: 26350965
- PMCID: PMC4600105
- DOI: 10.1128/mBio.01155-15
Novel Approaches Reveal that Toxoplasma gondii Bradyzoites within Tissue Cysts Are Dynamic and Replicating Entities In Vivo
Abstract
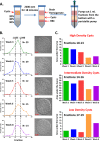
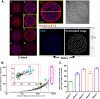
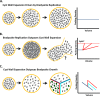
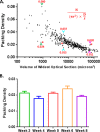
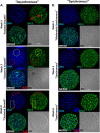
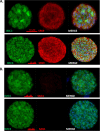
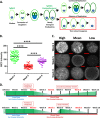
Despite their critical role in chronic toxoplasmosis, the biology of Toxoplasma gondii bradyzoites is poorly understood. In an attempt to address this gap, we optimized approaches to purify tissue cysts and analyzed the replicative potential of bradyzoites within these cysts. In order to quantify individual bradyzoites within tissue cysts, we have developed imaging software, BradyCount 1.0, that allows the rapid establishment of bradyzoite burdens within imaged optical sections of purified tissue cysts. While in general larger tissue cysts contain more bradyzoites, their relative "occupancy" was typically lower than that of smaller cysts, resulting in a lower packing density. The packing density permits a direct measure of how bradyzoites develop within cysts, allowing for comparisons across progression of the chronic phase. In order to capture bradyzoite endodyogeny, we exploited the differential intensity of TgIMC3, an inner membrane complex protein that intensely labels newly formed/forming daughters within bradyzoites and decays over time in the absence of further division. To our surprise, we were able to capture not only sporadic and asynchronous division but also synchronous replication of all bradyzoites within mature tissue cysts. Furthermore, the time-dependent decay of TgIMC3 intensity was exploited to gain insights into the temporal patterns of bradyzoite replication in vivo. Despite the fact that bradyzoites are considered replicatively dormant, we find evidence for cyclical, episodic bradyzoite growth within tissue cysts in vivo. These findings directly challenge the prevailing notion of bradyzoites as dormant nonreplicative entities in chronic toxoplasmosis and have implications on our understanding of this enigmatic and clinically important life cycle stage.
Importance: The protozoan Toxoplasma gondii establishes a lifelong chronic infection mediated by the bradyzoite form of the parasite within tissue cysts. Technical challenges have limited even the most basic studies on bradyzoites and the tissue cysts in vivo. Bradyzoites, which are viewed as dormant, poorly replicating or nonreplicating entities, were found to be surprisingly active, exhibiting not only the capacity for growth but also previously unrecognized patterns of replication that point to their being considerably more dynamic than previously imagined. These newly revealed properties force us to reexamine the most basic questions regarding bradyzoite biology and the progression of the chronic phase of toxoplasmosis. By developing new tools and approaches to study the chronic phase at the level of bradyzoites, we expose new avenues to tackle both drug development and a better understanding of events that may lead to reactivated symptomatic disease.
Copyright © 2015 Watts et al.
Figures



Comment in
-
A Bradyzoite is a Bradyzoite is a Bradyzoite?Trends Parasitol. 2015 Dec;31(12):610-612. doi: 10.1016/j.pt.2015.10.005. Epub 2015 Oct 26. Trends Parasitol. 2015. PMID: 26515047 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
