Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation
- PMID: 26351298
- PMCID: PMC4635118
- DOI: 10.1182/blood-2015-02-622233
Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation
Abstract
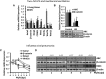
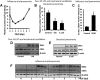
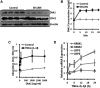
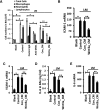
Neutrophil infiltration represents the early acute inflammatory response in acute lung injury. The recruitment of neutrophils from the peripheral blood across the endothelial-epithelial barrier into the alveolar airspace is highly regulated by the adhesion molecules on alveolar epithelial cells (AECs). Wnt/β-catenin signaling is involved in the progression of inflammatory lung diseases including asthma, emphysema, and pulmonary fibrosis. However, the function of Wnt/β-catenin signaling in acute lung inflammation is unknown. Here, we identified platelet-derived Dickkopf-1 (Dkk1) as the major Wnt antagonist contributing to the suppression of Wnt/β-catenin signaling in AECs during acute lung inflammation. Intratracheal administration of Wnt3a or an antibody capable of neutralizing Dkk1 inhibited neutrophil influx into the alveolar airspace of injured lungs. Activation of Wnt/β-catenin signaling in AECs attenuated intercellular adhesion molecule 1 (ICAM-1)/vascular cell adhesion molecule 1 (VCAM-1)-mediated adhesion of both macrophages and neutrophils to AECs. Our results suggest a role for Wnt/β-catenin signaling in modulating the inflammatory response, and a functional communication between platelets and AECs during acute lung inflammation. Targeting Wnt/β-catenin signaling and the communication between platelets and AECs therefore represents potential therapeutic strategies to limit the damage of acute pulmonary inflammation.
© 2015 by The American Society of Hematology.
Figures

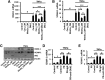
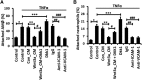
Comment in
-
Platelets promote pulmonary pull of polys.Blood. 2015 Nov 5;126(19):2174-5. doi: 10.1182/blood-2015-09-670455. Blood. 2015. PMID: 26542252 Free PMC article.
References
-
- Caudrillier A, Looney MR. Platelet-neutrophil interactions as a target for prevention and treatment of transfusion-related acute lung injury. Curr Pharm Des. 2012;18(22):3260–3266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

