Randomized, Placebo-Controlled, Phase III Trial of Yeast-Derived Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Versus Peptide Vaccination Versus GM-CSF Plus Peptide Vaccination Versus Placebo in Patients With No Evidence of Disease After Complete Surgical Resection of Locally Advanced and/or Stage IV Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697)
- PMID: 26351350
- PMCID: PMC4669592
- DOI: 10.1200/JCO.2015.62.0500
Randomized, Placebo-Controlled, Phase III Trial of Yeast-Derived Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Versus Peptide Vaccination Versus GM-CSF Plus Peptide Vaccination Versus Placebo in Patients With No Evidence of Disease After Complete Surgical Resection of Locally Advanced and/or Stage IV Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697)
Abstract
Purpose: We conducted a double-blind, placebo-controlled trial to evaluate the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) and peptide vaccination (PV) on relapse-free survival (RFS) and overall survival (OS) in patients with resected high-risk melanoma.
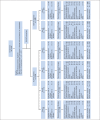
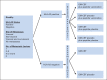
Patients and methods: Patients with completely resected stage IV or high-risk stage III melanoma were grouped by human leukocyte antigen (HLA) -A2 status. HLA-A2-positive patients were randomly assigned to receive GM-CSF, PV, both, or placebo; HLA-A2-negative patients, GM-CSF or placebo. Treatment lasted for 1 year or until recurrence. Efficacy analyses were conducted in the intent-to-treat population.
Results: A total of 815 patients were enrolled. There were no significant improvements in OS (stratified log-rank P = .528; hazard ratio, 0.94; 95% repeated CI, 0.77 to 1.15) or RFS (P = .131; hazard ratio, 0.88; 95% CI, 0.74 to 1.04) in the patients assigned to GM-CSF (n = 408) versus those assigned to placebo (n = 407). The median OS times with GM-CSF versus placebo treatments were 69.6 months (95% CI, 53.4 to 83.5 months) versus 59.3 months (95% CI, 44.4 to 77.3 months); the 5-year OS probability rates were 52.3% (95% CI, 47.3% to 57.1%) versus 49.4% (95% CI, 44.3% to 54.3%), respectively. The median RFS times with GM-CSF versus placebo were 11.4 months (95% CI, 9.4 to 14.8 months) versus 8.8 months (95% CI, 7.5 to 11.2 months); the 5-year RFS probability rates were 31.2% (95% CI, 26.7% to 35.9%) versus 27.0% (95% CI, 22.7% to 31.5%), respectively. Exploratory analyses showed a trend toward improved OS in GM-CSF-treated patients with resected visceral metastases. When survival in HLA-A2-positive patients who received PV versus placebo was compared, RFS and OS were not significantly different. Treatment-related grade 3 or greater adverse events were similar between GM-CSF and placebo groups.
Conclusion: Neither adjuvant GM-CSF nor PV significantly improved RFS or OS in patients with high-risk resected melanoma. Exploratory analyses suggest that GM-CSF may be beneficial in patients with resected visceral metastases; this observation requires prospective validation.
Trial registration: ClinicalTrials.gov NCT01989572.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures


References
-
- Lawson D, Kirkwood JM. Granulocyte-macrophage colony-stimulating factor: Another cytokine with adjuvant therapeutic benefit in melanoma? J Clin Oncol. 2000;18:1603–1605. - PubMed
-
- Wing EJ, Magee DM, Whiteside TL, et al. Recombinant human granulocyte/macrophage colony-stimulating factor enhances monocyte cytotoxicity and secretion of tumor necrosis factor alpha and interferon in cancer patients. Blood. 1989;73:643–646. - PubMed
-
- Grabstein KH, Urdal DL, Tushinski RJ, et al. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986;232:506–508. - PubMed
-
- Bell D, Young JW, Banchereau J. Advances in immunology. Adv Immunology. 1999;72:255–324. - PubMed
-
- Dong Z, Kumar R, Yang X, et al. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–810. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA20319/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- P50 CA121973/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- CA04326/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- CA180794/CA/NCI NIH HHS/United States
- CA180844/CA/NCI NIH HHS/United States
- U10 CA180864/CA/NCI NIH HHS/United States
- R01 CA168628/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA180802/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- U10 CA180854/CA/NCI NIH HHS/United States
- P30CA047904/CA/NCI NIH HHS/United States
- CA18082/CA/NCI NIH HHS/United States
- CA180854/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA180888/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- CA180864/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233184/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA180844/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

