Xanthine-based KMUP-1 improves HDL via PPARγ/SR-B1, LDL via LDLRs, and HSL via PKA/PKG for hepatic fat loss
- PMID: 26351364
- PMCID: PMC4617394
- DOI: 10.1194/jlr.M057547
Xanthine-based KMUP-1 improves HDL via PPARγ/SR-B1, LDL via LDLRs, and HSL via PKA/PKG for hepatic fat loss
Abstract
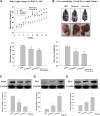
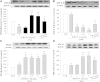
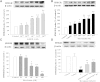
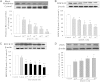
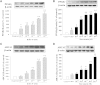
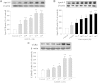
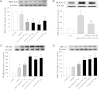
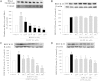
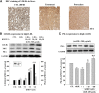
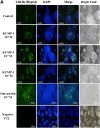
The phosphodiesterase inhibitor (PDEI)/eNOS enhancer KMUP-1, targeting G-protein coupled receptors (GPCRs), improves dyslipidemia. We compared its lipid-lowering effects with simvastatin and explored hormone-sensitive lipase (HSL) translocation in hepatic fat loss. KMUP-1 HCl (1, 2.5, and 5 mg/kg/day) and simvastatin (5 mg/kg/day) were administered in C57BL/6J male mice fed a high-fat diet (HFD) by gavage for 8 weeks. KMUP-1 inhibited HFD-induced plasma/liver TG, total cholesterol, and LDL; increased HDL/3-hydroxy-3-methylglutaryl-CoA reductase (HMGR)/Rho kinase II (ROCK II)/PPARγ/ABCA1; and decreased liver and body weight. KMUP-1 HCl in drinking water (2.5 mg/200 ml tap water) for 1-14 or 8-14 weeks decreased HFD-induced liver and body weight and scavenger receptor class B type I expression and increased protein kinase A (PKA)/PKG/LDLRs/HSL expression and immunoreactivity. In HepG2 cells incubated with serum or exogenous mevalonate, KMUP-1 (10(-7)∼10(-5) M) reversed HMGR expression by feedback regulation, colocalized expression of ABCA1/apolipoprotein A-I/LXRα/PPARγ, and reduced exogenous geranylgeranyl pyrophosphate/farnesyl pyrophosphate (FPP)-induced RhoA/ROCK II expression. A guanosine 3',5'-cyclic monophosphate (cGMP) antagonist reversed KMUP-1-induced ROCK II reduction, indicating cGMP/eNOS involvement. KMUP-1 inceased PKG and LDLRs surrounded by LDL and restored oxidized LDL-induced PKA expresion. Unlike simvastatin, KMUP-1 could not inhibit (14)C mevalonate formation. KMUP-1 could, but simvastatin could not, decrease ROCK II expression by exogenous FPP/CGPP. KMUP-1 improves HDL via PPARγ/LXRα/ABCA1/Apo-I expression and increases LDLRs/PKA/PKG/HSL expression and immunoreactivity, leading to TG hydrolysis to lower hepatic fat and body weight.
Keywords: 3-hydroxy-3-methylglutaryl-CoA reductase; endothelial nitric-oxide synthase/RhoA/Rho kinase II; low density lipoprotein/high density lipoprotein; protein kinase A/G; scavenger receptor class B type I/peroxisome proliferator activated receptor γ.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Theophylline-Based KMUP-1 Improves Steatohepatitis via MMP-9/IL-10 and Lipolysis via HSL/p-HSL in Obese Mice.Int J Mol Sci. 2016 Aug 17;17(8):1345. doi: 10.3390/ijms17081345. Int J Mol Sci. 2016. PMID: 27548140 Free PMC article.
-
KMUP-1, a GPCR Modulator, Attenuates Triglyceride Accumulation Involved MAPKs/Akt/PPARγ and PKA/PKG/HSL Signaling in 3T3-L1 Preadipocytes.Molecules. 2018 Sep 23;23(10):2433. doi: 10.3390/molecules23102433. Molecules. 2018. PMID: 30249030 Free PMC article.
-
NO donor KMUP-1 improves hepatic ischemia-reperfusion and hypoxic cell injury by inhibiting oxidative stress and pro-inflammatory signaling.Int J Immunopathol Pharmacol. 2013 Jan-Mar;26(1):93-106. doi: 10.1177/039463201302600109. Int J Immunopathol Pharmacol. 2013. PMID: 23527712
-
Foam cells in atherosclerosis.Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
-
Transcytosis of LDL Across Arterial Endothelium: Mechanisms and Therapeutic Targets.Arterioscler Thromb Vasc Biol. 2025 Apr;45(4):468-480. doi: 10.1161/ATVBAHA.124.321549. Epub 2025 Feb 27. Arterioscler Thromb Vasc Biol. 2025. PMID: 40013359 Free PMC article. Review.
Cited by
-
Theophylline-Based KMUP-1 Improves Steatohepatitis via MMP-9/IL-10 and Lipolysis via HSL/p-HSL in Obese Mice.Int J Mol Sci. 2016 Aug 17;17(8):1345. doi: 10.3390/ijms17081345. Int J Mol Sci. 2016. PMID: 27548140 Free PMC article.
-
KMUP-1, a GPCR Modulator, Attenuates Triglyceride Accumulation Involved MAPKs/Akt/PPARγ and PKA/PKG/HSL Signaling in 3T3-L1 Preadipocytes.Molecules. 2018 Sep 23;23(10):2433. doi: 10.3390/molecules23102433. Molecules. 2018. PMID: 30249030 Free PMC article.
-
Sesamol Alleviates Obesity-Related Hepatic Steatosis via Activating Hepatic PKA Pathway.Nutrients. 2020 Jan 26;12(2):329. doi: 10.3390/nu12020329. Nutrients. 2020. PMID: 31991934 Free PMC article.
-
Phosphodiesterase inhibitor KMUP-3 displays cardioprotection via protein kinase G and increases cardiac output via G-protein-coupled receptor agonist activity and Ca(2+) sensitization.Kaohsiung J Med Sci. 2016 Feb;32(2):55-67. doi: 10.1016/j.kjms.2016.01.005. Epub 2016 Feb 24. Kaohsiung J Med Sci. 2016. PMID: 26944323 Free PMC article.
References
-
- Beaudoin M. S., Graham T. E. 2011. Methylxanthines and human health: epidemiological and experimental evidence. Handb. Exp. Pharmacol. 200: 509–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

