Noonan syndrome and Turner syndrome patients respond similarly to 4 years' growth-hormone therapy: longitudinal analysis of growth-hormone-naïve patients enrolled in the NordiNet® International Outcome Study and the ANSWER Program
- PMID: 26351466
- PMCID: PMC4562101
- DOI: 10.1186/s13633-015-0015-1
Noonan syndrome and Turner syndrome patients respond similarly to 4 years' growth-hormone therapy: longitudinal analysis of growth-hormone-naïve patients enrolled in the NordiNet® International Outcome Study and the ANSWER Program
Abstract
Background: Turner syndrome (TS) and Noonan syndrome (NS) are distinct syndromes associated with short stature and other similar phenotypic features. We compared the responses to growth hormone (GH) therapy of TS and NS patients enrolled in the NordiNet® International Outcome Study (IOS) or the American Norditropin Studies: Web-Enabled Research (ANSWER) Program, which collect information on GH therapy in clinical practice.
Methods: Repeated-measures regression analysis was performed on change in height standard deviation score (HSDS) and target-height-corrected HSDS, based on national normal references and treatment-naïve disease-specific references. Models were adjusted for baseline age and HSDS, and average GH dose. The study population was paediatric patients with TS and NS in the NordiNet® IOS and ANSWER Program. Longitudinal growth responses over 4 years were evaluated.
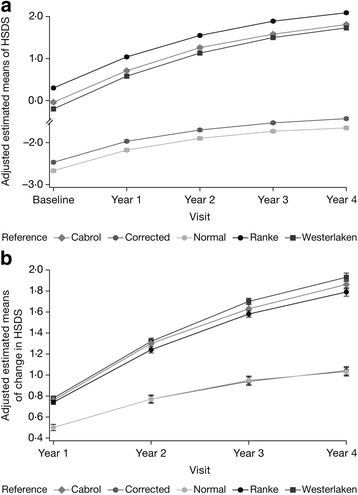
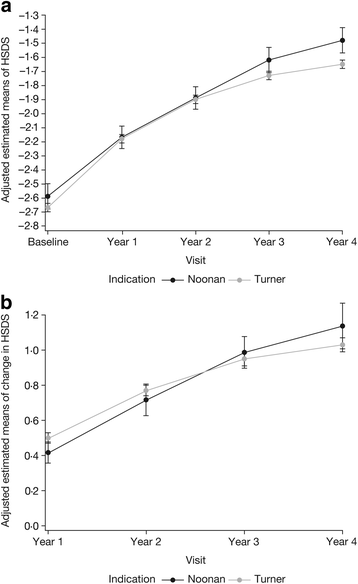
Results: In 30 NS patients (24 males; baseline age 8.39 ± 3.45 years) and 294 TS patients (7.81 ± 3.22 years), 4-year adjusted ΔHSDS were +1.14 ± 0.13 and +1.03 ± 0.04, respectively (national references). Based on untreated, disease-specific references, 4-year adjusted ΔHSDS for NS and TS were +1.48 ± 0.10 and +1.79 ± 0.04. The analyses showed a significant increase in HSDS over time for both NS and TS (P < 0.0001). ΔHSDS in NS was higher with younger baseline age; ΔHSDS in TS was higher for patients with younger baseline age and higher GH dose.
Conclusions: NS and TS patients responded well and similarly over 4 years of GH treatment.
Keywords: Human growth hormone; Noonan syndrome; Turner syndrome.
Figures

References
-
- Baxter L, Bryant J, Cave CB, Milne R. Recombinant growth hormone for children and adolescents with Turner syndrome. Cochrane Database Syst Rev. 2007:CD003887. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

