Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling
- PMID: 26351666
- PMCID: PMC4586849
- DOI: 10.1073/pnas.1509929112
Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling
Abstract
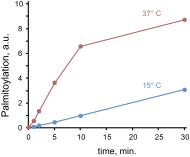
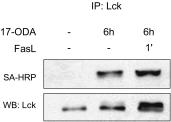
Palmitoylation is the posttranslational modification of proteins with a 16-carbon fatty acid chain through a labile thioester bond. The reversibility of protein palmitoylation and its profound effect on protein function suggest that this modification could play an important role as an intracellular signaling mechanism. Evidence that palmitoylation of proteins occurs with the kinetics required for signal transduction is not clear, however. Here we show that engagement of the Fas receptor by its ligand leads to an extremely rapid and transient increase in palmitoylation levels of the tyrosine kinase Lck. Lck palmitoylation kinetics are consistent with the activation of downstream signaling proteins, such as Zap70 and PLC-γ1. Inhibiting Lck palmitoylation not only disrupts proximal Fas signaling events, but also renders cells resistant to Fas-mediated apoptosis. Knockdown of the palmitoyl acyl transferase DHHC21 eliminates activation of Lck and downstream signaling after Fas receptor stimulation. Our findings demonstrate highly dynamic Lck palmitoylation kinetics that are essential for signaling downstream of the Fas receptor.
Keywords: Fas; Lck; apoptosis; calcium; protein palmitoylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Linder ME, Deschenes RJ. Palmitoylation: Policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. - PubMed
-
- Baekkeskov S, Kanaani J. Palmitoylation cycles and regulation of protein function (review) Mol Membr Biol. 2009;26(1):42–54. - PubMed
-
- Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761(4):474–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

