Transcellular spreading of huntingtin aggregates in the Drosophila brain
- PMID: 26351672
- PMCID: PMC4593132
- DOI: 10.1073/pnas.1516217112
Transcellular spreading of huntingtin aggregates in the Drosophila brain
Abstract
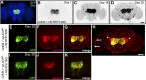
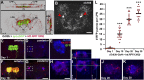
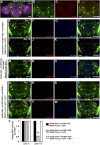
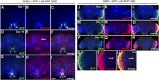
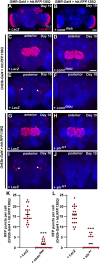
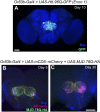
A key feature of many neurodegenerative diseases is the accumulation and subsequent aggregation of misfolded proteins. Recent studies have highlighted the transcellular propagation of protein aggregates in several major neurodegenerative diseases, although the precise mechanisms underlying this spreading and how it relates to disease pathology remain unclear. Here we use a polyglutamine-expanded form of human huntingtin (Htt) with a fluorescent tag to monitor the spreading of aggregates in the Drosophila brain in a model of Huntington's disease. Upon expression of this construct in a defined subset of neurons, we demonstrate that protein aggregates accumulate at synaptic terminals and progressively spread throughout the brain. These aggregates are internalized and accumulate within other neurons. We show that Htt aggregates cause non-cell-autonomous pathology, including loss of vulnerable neurons that can be prevented by inhibiting endocytosis in these neurons. Finally we show that the release of aggregates requires N-ethylmalemide-sensitive fusion protein 1, demonstrating that active release and uptake of Htt aggregates are important elements of spreading and disease progression.
Keywords: Huntington's disease; disease model; expanded triplet repeat; neurodegeneration; transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. - PubMed
-
- Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. - PubMed
-
- Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110(5):517–536. - PubMed
-
- Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: Rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68(19):1576–1582. - PubMed
-
- Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68(19):1571–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

