Hyperglycemia impairs left-right axis formation and thereby disturbs heart morphogenesis in mouse embryos
- PMID: 26351675
- PMCID: PMC4586861
- DOI: 10.1073/pnas.1504529112
Hyperglycemia impairs left-right axis formation and thereby disturbs heart morphogenesis in mouse embryos
Abstract
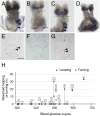
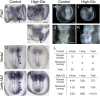
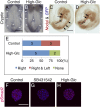
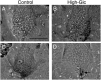
Congenital heart defects with heterotaxia are associated with pregestational diabetes mellitus. To provide insight into the mechanisms underlying such diabetes-related heart defects, we examined the effects of high-glucose concentrations on formation of the left-right axis in mouse embryos. Expression of Pitx2, which plays a key role in left-right asymmetric morphogenesis and cardiac development, was lost in the left lateral plate mesoderm of embryos of diabetic dams. Embryos exposed to high-glucose concentrations in culture also failed to express Nodal and Pitx2 in the left lateral plate mesoderm. The distribution of phosphorylated Smad2 revealed that Nodal activity in the node was attenuated, accounting for the failure of left-right axis formation. Consistent with this notion, Notch signal-dependent expression of Nodal-related genes in the node was also down-regulated in association with a reduced level of Notch signaling, suggesting that high-glucose concentrations impede Notch signaling and thereby hinder establishment of the left-right axis required for heart morphogenesis.
Keywords: congenital heart defects; diabetes; heterotaxia; left–right axis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Development: Maternal hyperglycaemia affects cardiac development.Nat Rev Endocrinol. 2015 Dec;11(12):689. doi: 10.1038/nrendo.2015.172. Epub 2015 Sep 29. Nat Rev Endocrinol. 2015. PMID: 26416218 No abstract available.
References
-
- Liu S, et al. Canadian Perinatal Surveillance System (Public Health Agency of Canada) Association between maternal chronic conditions and congenital heart defects: A population-based cohort study. Circulation. 2013;128(6):583–589. - PubMed
-
- Dolk H, Loane M, Garne E. European Surveillance of Congenital Anomalies (EUROCAT) Working Group Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123(8):841–849. - PubMed
-
- Jenkins KJ, et al. American Heart Association Council on Cardiovascular Disease in the Young Noninherited risk factors and congenital cardiovascular defects: Current knowledge: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: Endorsed by the American Academy of Pediatrics. Circulation. 2007;115(23):2995–3014. - PubMed
-
- Cockroft DL, Coppola PT. Teratogenic effects of excess glucose on head-fold rat embryos in culture. Teratology. 1977;16(2):141–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

