Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine
- PMID: 26351680
- PMCID: PMC4586868
- DOI: 10.1073/pnas.1514418112
Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine
Abstract
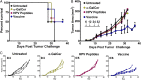
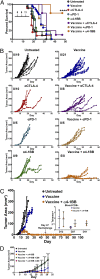
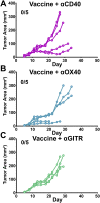
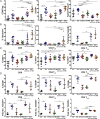
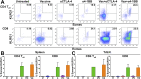
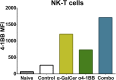
Antibody modulation of T-cell coinhibitory (e.g., CTLA-4) or costimulatory (e.g., 4-1BB) receptors promotes clinical responses to a variety of cancers. Therapeutic cancer vaccination, in contrast, has produced limited clinical benefit and no curative therapies. The E6 and E7 oncoproteins of human papilloma virus (HPV) drive the majority of genital cancers, and many oropharyngeal tumors. We discovered 15-19 amino acid peptides from HPV-16 E6/E7 for which induction of T-cell immunity correlates with disease-free survival in patients treated for high-grade cervical neoplasia. We report here that intranasal vaccination with these peptides and the adjuvant alpha-galactosylceramide elicits systemic and mucosal T-cell responses leading to reduced HPV(+) TC-1 tumor growth and prolonged survival in mice. We hypothesized that the inability of these T cells to fully reject established tumors resulted from suppression in the tumor microenvironment which could be ameliorated through checkpoint modulation. Combining this E6/E7 peptide vaccine with checkpoint blockade produced only modest benefit; however, coadministration with a 4-1BB agonist antibody promoted durable regression of established genital TC-1 tumors. Relative to other therapies tested, this combination of vaccine and α4-1BB promoted the highest CD8(+) versus regulatory FoxP3(+) T-cell ratios, elicited 2- to 5-fold higher infiltration by E7-specific CTL, and evoked higher densities of highly cytotoxic TcEO (T cytotoxic Eomesodermin) CD8 (>70-fold) and ThEO (T helper Eomesodermin) CD4 (>17-fold) T cells. These findings have immediate clinical relevance both in terms of the direct clinical utility of the vaccine studied and in illustrating the potential of 4-1BB antibody to convert therapeutic E6/E7 vaccines already in clinical trials into curative therapies.
Keywords: 4-1BB; HPV; cancer vaccine; checkpoint blockade; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






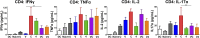
Similar articles
-
Mucosal HPV E6/E7 Peptide Vaccination in Combination with Immune Checkpoint Modulation Induces Regression of HPV+ Oral Cancers.Cancer Res. 2018 Sep 15;78(18):5327-5339. doi: 10.1158/0008-5472.CAN-18-0892. Epub 2018 Jul 27. Cancer Res. 2018. PMID: 30054333 Free PMC article.
-
An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression.Cancer Gene Ther. 2015 Sep;22(9):454-62. doi: 10.1038/cgt.2015.40. Epub 2015 Sep 4. Cancer Gene Ther. 2015. PMID: 26337747
-
Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection.Vaccine. 2007 Apr 30;25(17):3302-10. doi: 10.1016/j.vaccine.2007.01.010. Epub 2007 Jan 24. Vaccine. 2007. PMID: 17291642 Free PMC article.
-
Advances in peptide-based human papillomavirus therapeutic vaccines.Curr Top Med Chem. 2012;12(14):1581-92. doi: 10.2174/156802612802652402. Curr Top Med Chem. 2012. PMID: 22827526 Review.
-
The promise of combining cancer vaccine and checkpoint blockade for treating HPV-related cancer.Cancer Treat Rev. 2019 Aug;78:8-16. doi: 10.1016/j.ctrv.2019.07.001. Epub 2019 Jul 5. Cancer Treat Rev. 2019. PMID: 31302573 Free PMC article. Review.
Cited by
-
HPV-associated head and neck cancer is characterized by distinct profiles of CD8+ T cells and myeloid-derived suppressor cells.Cancer Immunol Immunother. 2023 Dec;72(12):4367-4383. doi: 10.1007/s00262-023-03571-8. Epub 2023 Nov 29. Cancer Immunol Immunother. 2023. PMID: 38019346 Free PMC article.
-
The Major Role of T Regulatory Cells in the Efficiency of Vaccination in General and Immunocompromised Populations: A Review.Vaccines (Basel). 2024 Aug 30;12(9):992. doi: 10.3390/vaccines12090992. Vaccines (Basel). 2024. PMID: 39340024 Free PMC article. Review.
-
Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer.J Immunother Cancer. 2019 Sep 18;7(1):252. doi: 10.1186/s40425-019-0728-4. J Immunother Cancer. 2019. PMID: 31533840 Free PMC article.
-
Cancer vaccines in the clinic.Bioeng Transl Med. 2023 Oct 27;9(1):e10588. doi: 10.1002/btm2.10588. eCollection 2024 Jan. Bioeng Transl Med. 2023. PMID: 38193112 Free PMC article. Review.
-
Peritumoral administration of immunomodulatory antibodies as a triple combination suppresses skin tumor growth without systemic toxicity.J Immunother Cancer. 2024 Jan 31;12(1):e007960. doi: 10.1136/jitc-2023-007960. J Immunother Cancer. 2024. PMID: 38296598 Free PMC article.
References
-
- Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80(6):827–841. - PubMed
-
- Moody CA, Laimins LA. Human papillomavirus oncoproteins: Pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. - PubMed
-
- Chaturvedi AK. Beyond cervical cancer: Burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4) Suppl:S20–S26. - PubMed
-
- Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: A potential clue to unlock cancer immunotherapy. Cancer J. 2010;16(4):399–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

