RNA transcription modulates phase transition-driven nuclear body assembly
- PMID: 26351690
- PMCID: PMC4586886
- DOI: 10.1073/pnas.1509317112
RNA transcription modulates phase transition-driven nuclear body assembly
Abstract
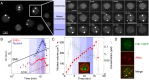
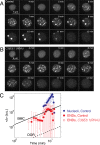
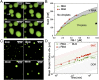
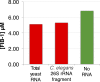
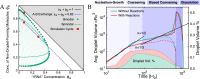
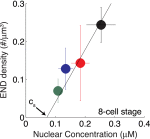
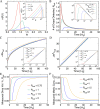
Nuclear bodies are RNA and protein-rich, membraneless organelles that play important roles in gene regulation. The largest and most well-known nuclear body is the nucleolus, an organelle whose primary function in ribosome biogenesis makes it key for cell growth and size homeostasis. The nucleolus and other nuclear bodies behave like liquid-phase droplets and appear to condense from the nucleoplasm by concentration-dependent phase separation. However, nucleoli actively consume chemical energy, and it is unclear how such nonequilibrium activity might impact classical liquid-liquid phase separation. Here, we combine in vivo and in vitro experiments with theory and simulation to characterize the assembly and disassembly dynamics of nucleoli in early Caenorhabditis elegans embryos. In addition to classical nucleoli that assemble at the transcriptionally active nucleolar organizing regions, we observe dozens of "extranucleolar droplets" (ENDs) that condense in the nucleoplasm in a transcription-independent manner. We show that growth of nucleoli and ENDs is consistent with a first-order phase transition in which late-stage coarsening dynamics are mediated by Brownian coalescence and, to a lesser degree, Ostwald ripening. By manipulating C. elegans cell size, we change nucleolar component concentration and confirm several key model predictions. Our results show that rRNA transcription and other nonequilibrium biological activity can modulate the effective thermodynamic parameters governing nucleolar and END assembly, but do not appear to fundamentally alter the passive phase separation mechanism.
Keywords: Brownian coalescence; Flory–Huggins regular solution theory; Ostwald ripening; RNA/protein droplets; intracellular phase separation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Brangwynne CP, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324(5935):1729–1732. - PubMed
-
- Wippich F, et al. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell. 2013;152(4):791–805. - PubMed
-
- Weber SC, Brangwynne CP. Getting RNA and protein in phase. Cell. 2012;149(6):1188–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

