Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry
- PMID: 26351694
- PMCID: PMC4603497
- DOI: 10.1073/pnas.1515606112
Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry
Abstract
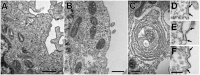
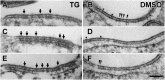
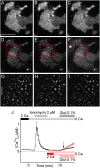
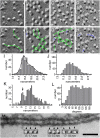
Stromal interacting molecule (STIM) and Orai proteins constitute the core machinery of store-operated calcium entry. We used transmission and freeze-fracture electron microscopy to visualize STIM1 and Orai1 at endoplasmic reticulum (ER)-plasma membrane (PM) junctions in HEK 293 cells. Compared with control cells, thin sections of STIM1-transfected cells possessed far more ER elements, which took the form of complex stackable cisternae and labyrinthine structures adjoining the PM at junctional couplings (JCs). JC formation required STIM1 expression but not store depletion, induced here by thapsigargin (TG). Extended molecules, indicative of STIM1, decorated the cytoplasmic surface of ER, bridged a 12-nm ER-PM gap, and showed clear rearrangement into small clusters following TG treatment. Freeze-fracture replicas of the PM of Orai1-transfected cells showed extensive domains packed with characteristic "particles"; TG treatment led to aggregation of these particles into sharply delimited "puncta" positioned upon raised membrane subdomains. The size and spacing of Orai1 channels were consistent with the Orai crystal structure, and stoichiometry was unchanged by store depletion, coexpression with STIM1, or an Orai1 mutation (L273D) affecting STIM1 association. Although the arrangement of Orai1 channels in puncta was substantially unstructured, a portion of channels were spaced at ∼15 nm. Monte Carlo analysis supported a nonrandom distribution for a portion of channels spaced at ∼15 nm. These images offer dramatic, direct views of STIM1 aggregation and Orai1 clustering in store-depleted cells and provide evidence for the interaction of a single Orai1 channel with small clusters of STIM1 molecules.
Keywords: Orai1; SOCE; STIM1; electron microscopy; nanoscale patterning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry.Biochem J. 2009 Dec 14;425(1):159-68. doi: 10.1042/BJ20090884. Biochem J. 2009. PMID: 19843011 Free PMC article.
-
Fast endocytic recycling determines TRPC1-STIM1 clustering in ER-PM junctions and plasma membrane function of the channel.Biochim Biophys Acta. 2015 Oct;1853(10 Pt A):2709-21. doi: 10.1016/j.bbamcr.2015.07.019. Epub 2015 Jul 30. Biochim Biophys Acta. 2015. PMID: 26232624
-
A relay mechanism between EB1 and APC facilitate STIM1 puncta assembly at endoplasmic reticulum-plasma membrane junctions.Cell Calcium. 2013 Sep;54(3):246-56. doi: 10.1016/j.ceca.2013.06.008. Epub 2013 Jul 18. Cell Calcium. 2013. PMID: 23871111
-
Remodelling of the endoplasmic reticulum during store-operated calcium entry.Biol Cell. 2011 Aug;103(8):365-80. doi: 10.1042/BC20100152. Biol Cell. 2011. PMID: 21736554 Review.
-
The STIM-Orai Pathway: Conformational Coupling Between STIM and Orai in the Activation of Store-Operated Ca2+ Entry.Adv Exp Med Biol. 2017;993:83-98. doi: 10.1007/978-3-319-57732-6_5. Adv Exp Med Biol. 2017. PMID: 28900910 Free PMC article. Review.
Cited by
-
Homeostatic regulation of the PI(4,5)P2-Ca(2+) signaling system at ER-PM junctions.Biochim Biophys Acta. 2016 Aug;1861(8 Pt B):862-873. doi: 10.1016/j.bbalip.2016.02.015. Epub 2016 Feb 24. Biochim Biophys Acta. 2016. PMID: 26924250 Free PMC article. Review.
-
Role of store-operated Ca2+ entry in cardiovascular disease.Cell Commun Signal. 2022 Mar 18;20(1):33. doi: 10.1186/s12964-022-00829-z. Cell Commun Signal. 2022. PMID: 35303866 Free PMC article. Review.
-
Increased Confinement and Polydispersity of STIM1 and Orai1 after Ca2+ Store Depletion.Biophys J. 2020 Jan 7;118(1):70-84. doi: 10.1016/j.bpj.2019.11.019. Epub 2019 Nov 22. Biophys J. 2020. PMID: 31818466 Free PMC article.
-
Intracellular Membrane Contact Sites in Skeletal Muscle Cells.Membranes (Basel). 2025 Jan 14;15(1):29. doi: 10.3390/membranes15010029. Membranes (Basel). 2025. PMID: 39852269 Free PMC article. Review.
-
Transverse tubule remodeling enhances Orai1-dependent Ca2+ entry in skeletal muscle.Elife. 2019 Oct 28;8:e47576. doi: 10.7554/eLife.47576. Elife. 2019. PMID: 31657717 Free PMC article.
References
-
- Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

