Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion
- PMID: 26352007
- PMCID: PMC4733851
- DOI: 10.1002/14651858.CD007324.pub3
Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion
Abstract
Background: Central retinal vein occlusion (CRVO) is a common retinal vascular abnormality associated with conditions such as hypertension, diabetes, glaucoma, and a wide variety of hematologic disorders. Macular edema (ME) represents an important vision-threatening complication of CRVO. Intravitreal steroids (IVS), such as triamcinolone acetonide, have been utilized to treat macular edema stemming from a variety of etiologies and may be a treatment option for CRVO-ME.
Objectives: To explore the effectiveness and safety of intravitreal steroids in the treatment of CRVO-ME.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014 Issue 10), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2014), EMBASE (January 1980 to November 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 13 November 2014. For all included primary studies, we used The Science Citation Index (3 December 2014) and manually reviewed reference lists to identify other possible relevant trials.
Selection criteria: We included randomized controlled trials (RCTs) that compared intravitreal steroids, of any dosage and duration of treatment of at least six months, with observation for the treatment of CRVO-ME.
Data collection and analysis: Two review authors independently screened titles and abstracts identified from the electronic searches and assessed full-text articles from potentially eligible trials. Two review authors independently assessed trial characteristics, risk of bias, and extracted data from included trials. We contacted investigators of included trials for desired data not provided in the trial reports.
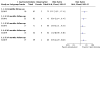
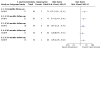
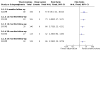
Main results: We included two RCTs that enrolled a total of 708 participants with CRVO-ME. SCORE compared triamcinolone acetonide intravitreal injections (n = 165) with observation (n = 72); GENEVA compared dexamethasone intravitreal implants (n = 290) with sham injections (n = 147). We observed characteristics indicative of high risk of bias due to incomplete outcome data in SCORE and selective outcome reporting in GENEVA. Loss to follow-up was high with 10% in the steroid groups and almost twice as much (17%) in the observation group. GENEVA enrolled participants with both branch and central retinal vein occlusion, but did not present subgroup data for the CRVO-ME population. A qualitative assessment of the results from GENEVA indicated that the dexamethasone implant was not associated with improvement in visual acuity after six months among participants with CRVO-ME. Although the SCORE investigators reported that participants treated with 1 mg (n = 82) or 4 mg (n = 83) triamcinolone intravitreal injections were five times more likely to have gained 15 letters or more in visual acuity compared with participants in the observation group (1 mg; risk ratio (RR): 5.27; 95% confidence interval (CI) 1.62 to 17.15; 4 mg RR 4.92; 95% CI 1.50 to 16.10) by the eighth-month follow-up examination, the average visual acuity decreased in all three groups. However, eyes treated with triamcinolone lost fewer letters than participants in the observation group at 8 months (1 mg mean difference (MD): 8.70 letters, 95% CI 1.86 to 15.54; 4 mg MD: 9.80 letters, 95% CI 3.32 to 16.28). A higher incidence of adverse events was noted with IVS therapy when compared with observation alone. As many as 20% to 35% of participants experienced an adverse event in the IVS groups compared with 8% of participants in the observation group of the SCORE study. The GENEVA investigators reported 63% in the treatment arm versus 43% in the observation arm experienced an adverse event. The most commonly encountered adverse events were elevated intraocular pressure, progression of cataracts, and retinal neovascularization. We graded the quality of evidence as low due to study limitations, imprecision of treatment estimates, and selective outcome reporting.
Authors' conclusions: The two RCTs reviewed herein provide insufficient evidence to determine the benefits of IVS for individuals with CRVO-ME. The improvement in visual acuity noted in the SCORE trial should be interpreted with caution as outcome data were missing for a large proportion of the observation group. Adverse events were observed more often with IVS treatment compared with observation/no treatment.
Conflict of interest statement
DG, PBG, KM: none known.
Figures
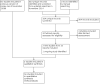




Update of
-
Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD007324. doi: 10.1002/14651858.CD007324.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2015 Sep 09;(9):CD007324. doi: 10.1002/14651858.CD007324.pub3. PMID: 19160332 Free PMC article. Updated.
References
References to studies included in this review
GENEVA {published data only}
-
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011;118(12):2453-60. - PubMed
-
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117(6):1134-46. - PubMed
-
- Haller JA, Blumenkranz MS, Williams GA, Kuppermann BD. Treatment of persistent macular edema associated with central and branch retinal vein occlusion with extended delivery of intravitreal dexamethasone. Investigative Ophthalmology and Visual Science 2003;44(2):ARVO E-abstract 4311.
-
- Kuppermann BD, Haller JA, Bandello F, Loewenstein A, Jiao J, Li XY, et al. Onset and duration of visual acuity improvement after dexamethasone intravitreal implant in eyes with macular edema due to retinal vein occlusion. Retina 2014;34(9):1743-9. - PubMed
-
- Yeh WS, Haller JA, Lanzetta P, Kuppermann BD, Wong TY, Mitchell P, et al. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology 2012;119(6):1190-8. - PubMed
SCORE {published data only}
-
- Blodi BA, Domalpally A, Scott IU, Ip MS, Oden NL, Elledge J, et al. Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study system for evaluation of stereoscopic color fundus photographs and fluorescein angiograms: SCORE study report 9. Archives of Ophthalmology 2010;128(9):1140-5. - PMC - PubMed
-
- Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Archives of Ophthalmology 2009;127(9):1101-14. - PMC - PubMed
-
- Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Erratum. Archives of Ophthalmology 2009;127(12):1648. - PMC - PubMed
References to studies excluded from this review
Bashshur 2004 {published data only}
-
- Bashshur ZF, Ma'luf RN, Allam S, Jurdi FA, Haddad RS, Noureddin BN. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Archives of Ophthalmology 2004;122(8):1137-40. - PubMed
Batioglu 2007 {published data only}
-
- Batioglu F, Ozmert E, Akmese E. Two-year results of intravitreal triamcinolone acetonide injection for the treatment of macular edema due to central retinal vein occlusion. Annals of Ophthalmology 2007;39(4):307-12. - PubMed
Cheng 2009 {published data only}
-
- Cheng KC, Wu WC, Lin CJ. Intravitreal triamcinolone acetonide for patients with macular oedema due to central retinal vein occlusion in Taiwan. Eye 2009;23(4):849-57. - PubMed
Chuang 2010 {published data only}
-
- Chuang LH, Yeung L, Wang NK, Chen HS, Ku WC, Lai CC. Secondary ocular hypertension after intravitreal injection with 2 mg or 4 mg of triamcinolone in retinal vein occlusion. Journal of Ocular Pharmacology and Therapeutics 2010;26(4):325-8. - PubMed
Gelston 2006 {published data only}
-
- Gelston CD, Olson JL, Mandava N. Macular oedema in central retinal vein occlusion treated with intravitreal triamcinolone. Acta Ophthalmologica Scandinavica 2006;84(3):314-8. - PubMed
Georgopoulos 2006 {published data only}
-
- Georgopoulos M, Sacu S, Vecsei PV, Michels S, Kiss C, Scholda C, et al. Therapy of macular edema with an intravitreal dexamethasone implant. Spektrum Der Augenheilkunde 2006;20(5):231-3.
Jain 2012 {published data only}
-
- Jain N, Stinnet S, Jaffe GJ. Prospective study of a fluocinolone acetonide implant for chronic macular edema from central retinal vein occlusion: thirty-six-month results. Ophthalmology 2012;119(1):132-7. - PubMed
Jiang 2006 {published data only}
-
- Jiang Y, Wang K, Li X. Clinical observation of intravitreal injection of triamcinolone acetonide for macular edema secondary to retinal vein occlusion. Chinese Ophthalmic Research 2006;24(6):639-42.
Jonas 2005a {published data only}
-
- Jonas JB, Akkoyun I, Kamppeter B, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for treatment of central retinal vein occlusion. European Journal of Ophthalmology 2005;15(6):751-8. - PubMed
Manaviat 2008 {published data only}
-
- Manaviat MR, Rashidi M. Intravitreal triamcinolone acetonide for macular edema in retinal vein occlusion. International Journal of Ophthalmology 2008;8(2):230-3.
Muni 2010 {published data only}
-
- Muni RH, Kertes PJ, Kohly RP. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care versus Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5: comment. Evidence-Based Ophthalmology 2010;11(1):13-5.
Ramezani 2006 {published data only}
-
- Ramezani A, Entezari M, Moradian S, Tabatabaei H, Kadkhodaei S. Intravitreal triamcinolone for acute central retinal vein occlusion; a randomized clinical trial. Graefes Archive for Clinical and Experimental Ophthalmology 2006;244(12):1601-6. - PubMed
ROVO {published data only}
-
- Aggermann T, Brunner S, Krebs I, Haas P, Womastek I, Brannath W, et al. A prospective, randomised, multicenter trial for surgical treatment of central retinal vein occlusion: results of the Radial Optic Neurotomy for Central Vein Occlusion (ROVO) study group. Graefe's Archive for Clinical and Experimental Ophthalmology 2013;251(4):1165-72. - PubMed
References to ongoing studies
NCT01660802 {unpublished data only}
-
- NCT01660802. Safety and efficacy study of dexamethasone in the treatment of patients with macular edema following retinal vein occlusion (RVO). clinicaltrials.gov/ct2/show/NCT01660802 (accessed 3 February 2014).
Additional references
Aggermann 2006
-
- Aggermann T, Stolba U, Brunner S, Binder S. Endophthalmitis with retinal necrosis following intravitreal triamcinolone acetonide injection. Ophthalmologica 2006;220(2):131-3. - PubMed
Al‐Dhibi 2007
Antcliff 2001
-
- Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J. Intravitreal triamcinolone for uveitic cystoid macular oedema: an optical coherence tomography study. Ophthalmology 2001;108(4):765-72. - PubMed
Bakri 2012
-
- Bakri SJ, Omar AF. Evolution of vitreomacular traction following the use of the dexamethasone intravitreal implant (Ozurdex) in the treatment of macular edema secondary to central retinal vein occlusion. Journal of Ocular Pharmacology and Therapeutics 2012;28(5):547-9. - PubMed
Benz 2003
-
- Benz MS, Murray TG, Dubovy SR, Katz RS, Eifrig CW. Endophthalmitis caused by mycobacterium chelonae abscessus after intravitreal injection of triamcinolone. Archives of Ophthalmology 2003;121(2):271-3. - PubMed
Braithwaite 2014
Clemens 2013
-
- Clemens CR, Bertelmann T, Meyer CH. Vitreous traction after Ozurdex injection. Journal of Ocular Pharmacology and Therapeutics 2013;29(1):3-4. - PubMed
Conway 2003
-
- Conway MD, Canakis C, Livir-Rallatos C, Peyman GA. Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular oedema. Journal of Cataract and Refractive Surgery 2003;29(1):27-33. - PubMed
COPERNICUS 2013
-
- Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. American Journal of Ophthalmology 2013;155(3):429-37. - PubMed
CRUISE 2007
-
- Varma R, Bressler NM, Suñer I, Lee P, Dolan CM, Ward J, et al. Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials. Ophthalmology 2012;119(10):2108-18. - PubMed
CVOS 1995
-
- The Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology 1995;102(10):1425-33. - PubMed
CVOS 1997
-
- The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Archives of Ophthalmology 1997;115(4):486-91. - PubMed
Eye Disease Case‐Control Study 1996
-
- The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Archives of Ophthalmology 1996;114(5):545-54. - PubMed
GALILEO 2014
-
- Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology 2014;121(1):202-8. - PubMed
Glanville 2006
Goff 2006
-
- Goff MJ, Jumper JM, Yang SS, Fu AD, Johnson RN, McDonald HR, et al. Intravitreal triamcinolone acetonide treatment of macular edema associated with central retinal vein occlusion. Retina 2006;26(8):896-901. - PubMed
Green 1981
Gregori 2006
-
- Gregori NZ, Rosenfeld PJ, Puliafito CA, Flynn HW Jr, Lee JE, Mavrofrides EC, et al. One-year safety and efficacy of intravitreal triamcinolone acetonide for the management of macular edema secondary to central retinal vein occlusion. Retina 2006;26(8):889-95. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Stern JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hirasawa 2009
-
- Hirasawa M, Noda K, Shinoda H, Ozawa Y, Tsubota K, Ishida S. Secondary macular hole associated with central retinal vein occlusion treated with corticosteroid injection. Japanese Journal of Ophthalmology 2009;53(3):279-81. - PubMed
Ip 2002
-
- Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Archives of Ophthalmology 2002;120(9):1217-9. - PubMed
Ip 2003
-
- Ip M, Kahana A, Altaweel M. Treatment of central retinal vein occlusion with triamcinolone acetonide: an optical coherence tomography study. Seminars in Ophthalmology 2003;18(2):67-73. - PubMed
Ip 2004
-
- Ip MS, Gottlieb JL, Kahana A, Scott IU, Altaweel MM, Blodi BA, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Archives of Ophthalmology 2004;122(8):1131-6. - PubMed
Jain 2006
-
- Jain A, Vishwanath MR, Charles SJ. Triamcinolone pseudo-cataract. Annals of Ophthalmology 2006;38(1):67-8. - PubMed
Jonas 2002
-
- Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular oedema in central retinal vein occlusion. Graefes Archive for Clinical and Experimental Ophthalmology 2002;240(9):782-3. - PubMed
Jonas 2005b
-
- Jonas JB. Intravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseases. Acta Ophthalmologica Scandinavica 2005;83(6):645-63. - PubMed
Joshi 2013
-
- Joshi L, Yaganti S, Gemenetzi M, Lightman S, Lindfield D, Liolios V, et al. Dexamethasone implants in retinal vein occlusion:12-month clinical effectiveness using repeat injections as-needed. British Journal of Ophthalmology Online 2013;97(8):1040-4. - PubMed
Karacorlu 2004
-
- Karacorlu M, Ozdemir H, Karacorlu S. Intravitreal triamcinolone acetonide for the treatment of central retinal vein occlusion in young patients. Retina 2004;24(2):324-7. - PubMed
Kaushik 2004
-
- Kaushik S, Gupta V, Gupta A, Dogra MR, Singh R. Intractable glaucoma following intravitreal triamcinolone in central retinal vein occlusion. American Journal of Ophthalmology 2004;137(4):758-60. - PubMed
Kivilcim 2000
-
- Kivilcim M, Peyman GA, El-Dessouky ES, Kazi AA, Cheema R, Hegazy H. Retinal toxicity of triamcinolone acetonide in silicone-filled eyes. Ophthalmic Surgery and Lasers 2000;31(6):474-8. - PubMed
Konstantopoulos 2007
-
- Konstantopoulos A, Williams CP, Newsom RS, Luff AJ. Ocular morbidity associated with intravitreal triamcinolone acetonide. Eye 2007;21(3):317-20. - PubMed
Krepler 2005
-
- Krepler K. Intravitreal triamcinolone acetonide in patients with macular oedema due to central retinal vein occlusion. Acta Ophthalmologica Scandinavica 2005;83(1):71-5. - PubMed
Lang 2007
-
- Lang Y, Zemel E, Miller B, Perlman I. Retinal toxicity of intravitreal Kenalog in albino rabbits. Retina 2007;27(6):778-88. - PubMed
Lattanzio 2007
-
- Lattanzio R, Ramoni A, Scotti F, Introini U. Macular hole and intravitreal injection of triamcinolone acetonide for macular edema due to central retinal vein occlusion. European Journal of Ophthalmology 2007;17(3):451-3. - PubMed
Mandelcorn 2007
-
- Mandelcorn MS, Mandelcorn E, Guan K, Adatia FA. Surgical macular decompression for macular edema in retinal vein occlusion. Canadian Journal of Ophthalmology 2007;42(1):116-22. - PubMed
Mohamed 2007
-
- Mohamed Q, McIntosh RL, Saw SM, Wong TY. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2007;114(3):507-19. - PubMed
Moschos 2007
-
- Moschos MM, Brouzas D, Loukianou E, Apostolopoulos M, Moschos M. Intraocular triamcinolone acetonide for macular edema due to CRVO. A multifocal-ERG and OCT study. Documenta Ophthalmologica 2007;114(1):1-7. - PubMed
Mushtaq 2009
-
- Mushtaq B, Gupta R, Elsherbiny S, Murray PI. Ocular syphilis unmasked following intravitreal triamcinolone injection. Ocular Immunology and Inflammation 2009;17(3):213-5. - PubMed
Nauck 1997
-
- Nauck M, Roth M, Tamm M, Eickelberg O, Wieland H, Stulz P, et al. Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. American Journal of Respiratory Cell and Molecular Biology 1997;16(4):398-406. - PubMed
Nauck 1998
-
- Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. European Journal of Pharmacology 1998;341(2-3):309-15. - PubMed
Nkeme 2006
-
- Nkeme J, Glacet-Bernard A, Gnikpingo K, Zourdani A, Mimoun G, Mahiddine H, et al. Surgical treatment of persistent macular edema in retinal vein occlusion. Journal Francais d Opthalmologie 2006;29(7):808-14. - PubMed
Ozdek 2005
-
- Ozdek SC, Aydin B, Gürelik G, Bahçeci U, Hasanreisoglu B. Effects of intravitreal triamcinolone injection on macular edema and visual prognosis in central retinal vein occlusion. International Ophthalmology 2005;26(1-2):27-34. - PubMed
Paques 2005
-
- Paques M, Krivosic V, Girmens JF, Giraud C, Sahel J, Gaudric A. Decreased venous tortuosity associated with resolution of macular edema after intravitreal injection of triamcinolone. Retina 2005;25(8):1099-101. - PubMed
Pardo‐Lopez 2012
-
- Pardo-Lopez D, Frances-Munoz E, Gallego-Pinazo R, Diaz-Llopis M. Anterior chamber migration of dexamethasone intravitreal implant (Ozurdex(R)). Graefe's Archive for Clinical and Experimental Ophthalmology 2012;250(11):1703-4. - PubMed
Park 2003
-
- Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. American Journal of Ophthalmology 2003;136(3):419-25. - PubMed
Pe'er 1998
-
- Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 1998;105(3):412-6. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre. The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Roth 2008
-
- Roth DB, Realini T, Feuer WJ, Radhakrishnan R, Gloth J, Heimmel MR, et al. Short-term complications of intravitreal injection of triamcinolone acetonide. Retina 2008;28(1):66-70. - PubMed
Ruiz‐Morena 2007
-
- Ruiz-Moreno JM, Montero JA, Bayon A, Rueda J, Vidal M. Retinal toxicity of intravitreal triamcinolone acetonide at high doses in the rabbit. Experimental Eye Research 2007;84(2):342-8. - PubMed
Sekiryu 2008
-
- Sekiryu T, Iida T, Kaneko H, Saito M. Cytomegalovirus retinitis after intravitreal triamcinolone acetonide in an immunocompetent patient. Japanese Journal of Ophthalmology 2008;52:414-6. - PubMed
Shyangdan 2010
-
- Shyangdan D, Cummins E, Lois N, Royle P, Waugh N. Dexamethasone implants in the treatment of macular oedema due to retinal vein occlusion: a single technology appraisal. www.nice.org.uk/guidance/ta229/resources/guidance-dexamethasone-intravit... (accessed 5 May 2014).
Srinivasan 2005
-
- Srinivasan S, Prasad S. Conjunctival necrosis following intravitreal injection of triamcinolone acetonide. Cornea 2005;24(8):1027-8. - PubMed
Turaka 2013
-
- Turaka K, Kwong HM Jr, De Souza S. Intravitreal implant migration into anterior chamber in a post-vitrectomy eye with central retinal vein occlusion and persistent macular edema. Ophthalmic Surgery, Lasers and Imaging Retina 2013;44(2):196-7. - PubMed
Williamson 2005
-
- Williamson TH, O'Donnell A. Intravitreal triamcinolone acetonide for cystoid macular edema in nonischemic central retinal vein occlusion. American Journal of Ophthalmology 2005;139(5):860-6. - PubMed
Yang 2005
-
- Yang CS, Chen MJ, Chou CK, Hsu WM. Refractory severe ocular hypertension after intravitreal triamcinolone acetonide injection. Ophthalmologica 2005;219(6):413-5. - PubMed
References to other published versions of this review
Gewaily 2008
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

