Characterization of glucose-related metabolic pathways in differentiated rat oligodendrocyte lineage cells
- PMID: 26352325
- PMCID: PMC4832329
- DOI: 10.1002/glia.22900
Characterization of glucose-related metabolic pathways in differentiated rat oligodendrocyte lineage cells
Abstract
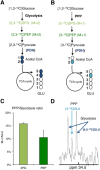
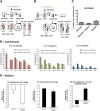
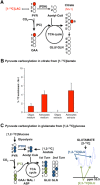
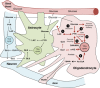
Although oligodendrocytes constitute a significant proportion of cells in the central nervous system (CNS), little is known about their intermediary metabolism. We have, therefore, characterized metabolic functions of primary oligodendrocyte precursor cell cultures at late stages of differentiation using isotope-labelled metabolites. We report that differentiated oligodendrocyte lineage cells avidly metabolize glucose in the cytosol and pyruvate derived from glucose in the mitochondria. The labelling patterns of metabolites obtained after incubation with [1,2-(13)C]glucose demonstrated that the pentose phosphate pathway (PPP) is highly active in oligodendrocytes (approximately 10% of glucose is metabolized via the PPP as indicated by labelling patterns in phosphoenolpyruvate). Mass spectrometry and magnetic resonance spectroscopy analyses of metabolites after incubation of cells with [1-(13)C]lactate or [1,2-(13)C]glucose, respectively, demonstrated that anaplerotic pyruvate carboxylation, which was thought to be exclusive to astrocytes, is also active in oligodendrocytes. Using [1,2-(13)C]acetate, we show that oligodendrocytes convert acetate into acetyl CoA which is metabolized in the tricarboxylic acid cycle. Analysis of labelling patterns of alanine after incubation of cells with [1,2-(13)C]acetate and [1,2-(13)C]glucose showed catabolic oxidation of malate or oxaloacetate. In conclusion, we report that oligodendrocyte lineage cells at late differentiation stages are metabolically highly active cells that are likely to contribute considerably to the metabolic activity of the CNS.
Keywords: 13C; acetate; energy metabolism; glucose; glycolysis; mitochondria; oligodendroglia; pyruvate carboxylation.
© 2015 The Authors. Glia Published by Wiley Periodicals, Inc.
Figures

References
-
- Almeida A, Delgado‐Esteban M, Bolanos JP, Medina JM. 2002. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem 81:207–217. - PubMed
-
- Amaral AI, Alves PM, Teixeira AP. 2014. Metabolic fux analysis tools to investigate brain metabolism in vitro In: Hirrlinger J, Waagepertersen HS, editor. Brain energy metabolism. New York: Humana Press; pp 107–144.
-
- Amaral AI, Teixeira AP, Martens S, Bernal V, Sousa MFQ, Alves PM. 2010. Metabolic alterations induced by ischemia in primary cultures of astrocytes: Merging 13C NMR spectroscopy and metabolic flux analysis. J Neurochem 113:735–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

