Structure of the toxic core of α-synuclein from invisible crystals
- PMID: 26352473
- PMCID: PMC4791177
- DOI: 10.1038/nature15368
Structure of the toxic core of α-synuclein from invisible crystals
Abstract
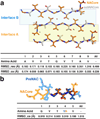
The protein α-synuclein is the main component of Lewy bodies, the neuron-associated aggregates seen in Parkinson disease and other neurodegenerative pathologies. An 11-residue segment, which we term NACore, appears to be responsible for amyloid formation and cytotoxicity of human α-synuclein. Here we describe crystals of NACore that have dimensions smaller than the wavelength of visible light and thus are invisible by optical microscopy. As the crystals are thousands of times too small for structure determination by synchrotron X-ray diffraction, we use micro-electron diffraction to determine the structure at atomic resolution. The 1.4 Å resolution structure demonstrates that this method can determine previously unknown protein structures and here yields, to our knowledge, the highest resolution achieved by any cryo-electron microscopy method to date. The structure exhibits protofibrils built of pairs of face-to-face β-sheets. X-ray fibre diffraction patterns show the similarity of NACore to toxic fibrils of full-length α-synuclein. The NACore structure, together with that of a second segment, inspires a model for most of the ordered portion of the toxic, full-length α-synuclein fibril, presenting opportunities for the design of inhibitors of α-synuclein fibrils.
Figures








Comment in
-
Parkinson's disease: Crystals of a toxic core.Nature. 2015 Sep 24;525(7570):458-9. doi: 10.1038/nature15630. Epub 2015 Sep 9. Nature. 2015. PMID: 26352470 No abstract available.
-
Invisible Killers.Mov Disord. 2016 Jan;31(1):44. doi: 10.1002/mds.26465. Epub 2016 Jan 9. Mov Disord. 2016. PMID: 26748962 No abstract available.
References
-
- Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9:13–24. - PubMed
-
- Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. - PubMed
-
- Krüger R. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. - PubMed
-
- Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
- R01 AG029430/AG/NIA NIH HHS/United States
- R01 GM102520/GM/NIGMS NIH HHS/United States
- P41 GM103403/GM/NIGMS NIH HHS/United States
- AG016570/AG/NIA NIH HHS/United States
- GM095887/GM/NIGMS NIH HHS/United States
- R01 GM095887/GM/NIGMS NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- GM102520/GM/NIGMS NIH HHS/United States
- 1R01-AG029430/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

