Targeting Mutant BRAF in Relapsed or Refractory Hairy-Cell Leukemia
- PMID: 26352686
- PMCID: PMC4811324
- DOI: 10.1056/NEJMoa1506583
Targeting Mutant BRAF in Relapsed or Refractory Hairy-Cell Leukemia
Abstract
Background: BRAF V600E is the genetic lesion underlying hairy-cell leukemia. We assessed the safety and activity of the oral BRAF inhibitor vemurafenib in patients with hairy-cell leukemia that had relapsed after treatment with a purine analogue or who had disease that was refractory to purine analogues.
Methods: We conducted two phase 2, single-group, multicenter studies of vemurafenib (at a dose of 960 mg twice daily)--one in Italy and one in the United States. The therapy was administered for a median of 16 weeks in the Italian study and 18 weeks in the U.S. study. Primary end points were the complete response rate (in the Italian trial) and the overall response rate (in the U.S. trial). Enrollment was completed (28 patients) in the Italian trial in April 2013 and is still open (26 of 36 planned patients) in the U.S. trial.
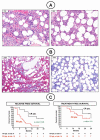
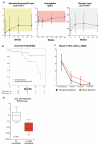
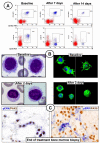
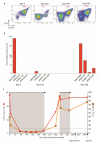
Results: The overall response rates were 96% (25 of 26 patients who could be evaluated) after a median of 8 weeks in the Italian study and 100% (24 of 24) after a median of 12 weeks in the U.S. study. The rates of complete response were 35% (9 of 26 patients) and 42% (10 of 24) in the two trials, respectively. In the Italian trial, after a median follow-up of 23 months, the median relapse-free survival was 19 months among patients with a complete response and 6 months among those with a partial response; the median treatment-free survival was 25 months and 18 months, respectively. In the U.S. trial, at 1 year, the progression-free survival rate was 73% and the overall survival rate was 91%. Drug-related adverse events were usually of grade 1 or 2, and the events most frequently leading to dose reductions were rash and arthralgia or arthritis. Secondary cutaneous tumors (treated with simple excision) developed in 7 of 50 patients. The frequent persistence of phosphorylated ERK-positive leukemic cells in bone marrow at the end of treatment suggests bypass reactivation of MEK and ERK as a resistance mechanism.
Conclusions: A short oral course of vemurafenib was highly effective in patients with relapsed or refractory hairy-cell leukemia. (Funded by the Associazione Italiana per la Ricerca sul Cancro and others; EudraCT number, 2011-005487-13; ClinicalTrials.gov number NCT01711632.).
Figures
References
-
- Foucar KFB, Catovsky D, Stein H. Hairy cell leukemia. In: Swerdlow SCE, Harris NL, et al., editors. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; Lyon, France: 2008. pp. 188–90.
-
- Tiacci E, Liso A, Piris M, Falini B. Evolving concepts in the pathogenesis of hairy-cell leukaemia. Nat Rev Cancer. 2006;6:437–48. - PubMed
-
- Kitagawa Y, Brahmachary M, Tiacci E, Dalla-Favera R, Falini B, Basso K. A microRNA signature specific for hairy cell leukemia and associated with modulation of the MAPK-JNK pathways. Leukemia. 2012;26:2564–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
