Certolizumab pegol plus methotrexate 5-year results from the rheumatoid arthritis prevention of structural damage (RAPID) 2 randomized controlled trial and long-term extension in rheumatoid arthritis patients
- PMID: 26353833
- PMCID: PMC4565002
- DOI: 10.1186/s13075-015-0767-2
Certolizumab pegol plus methotrexate 5-year results from the rheumatoid arthritis prevention of structural damage (RAPID) 2 randomized controlled trial and long-term extension in rheumatoid arthritis patients
Abstract
Introduction: As patients with rheumatoid arthritis (RA) receive treatment with anti-tumour necrosis factors over several years, it is important to evaluate their long-term safety and efficacy. The objective of this study was to examine the safety and benefits of certolizumab pegol (CZP)+methotrexate (MTX) treatment for almost 5 years in patients with RA.
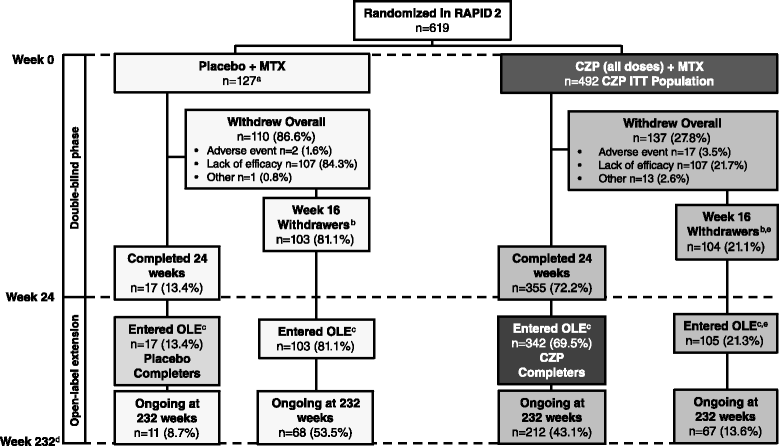
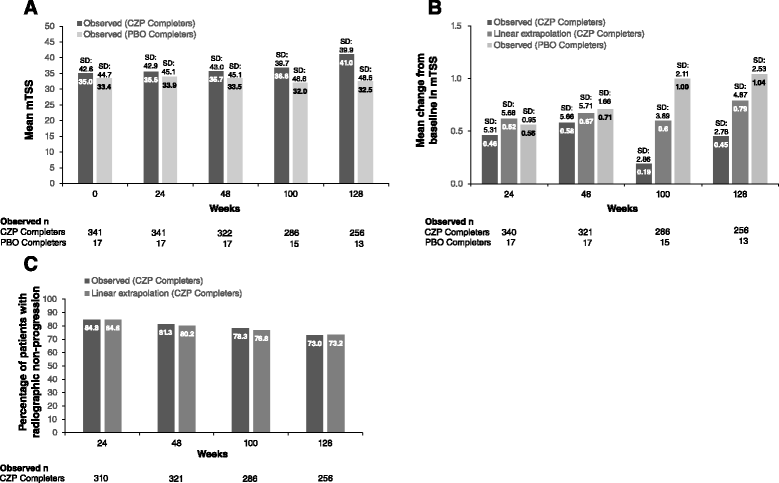
Methods: Patients who completed the 24-week Rheumatoid Arthritis Prevention of Structural Damage (RAPID) 2 randomized controlled trial (RCT; NCT00160602), or who were American College of Rheumatology (ACR) 20 non-responders at Week 16, entered the open-label extension (OLE; NCT00160641). After ≥6 months treatment with CZP 400 mg every two weeks (Q2W), dose was reduced to 200 mg Q2W, the approved maintenance dose. Safety data are presented from all patients who received ≥1 dose CZP (Safety population, n=612). Efficacy data are presented to Week 232 for the intent-to-treat (ITT, n=492) and Week 24 CZP RCT Completer (n=342) populations, and through 192 weeks of dose-reduction for the Dose-reduction population (patients whose CZP dose was reduced to 200 mg, n=369). Radiographic progression (modified total Sharp score change from RCT baseline >0.5) to Week 128 is reported for the Week 24 CZP Completers.
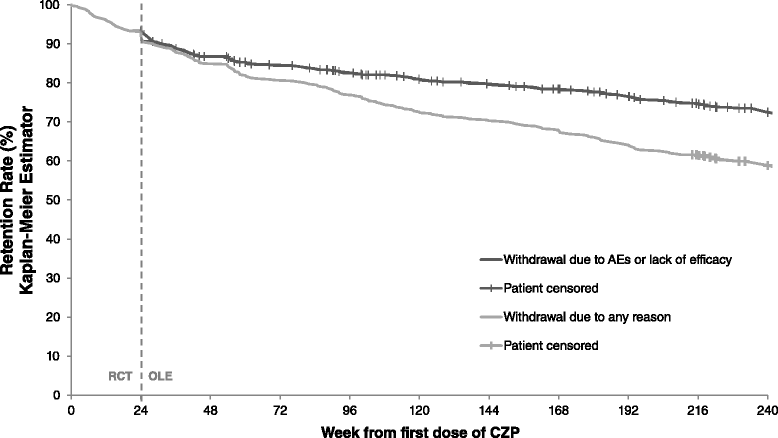
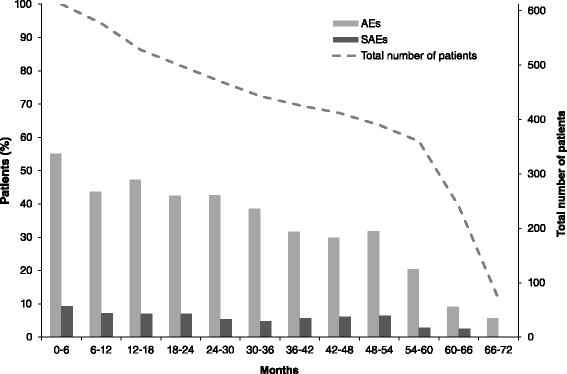
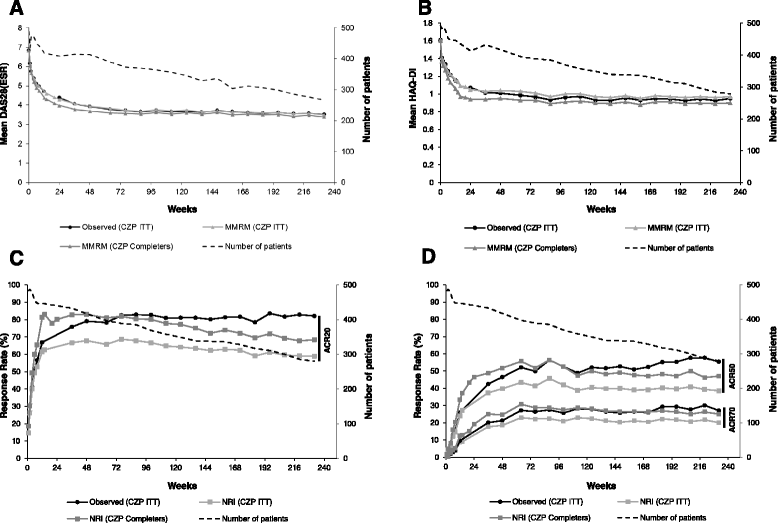
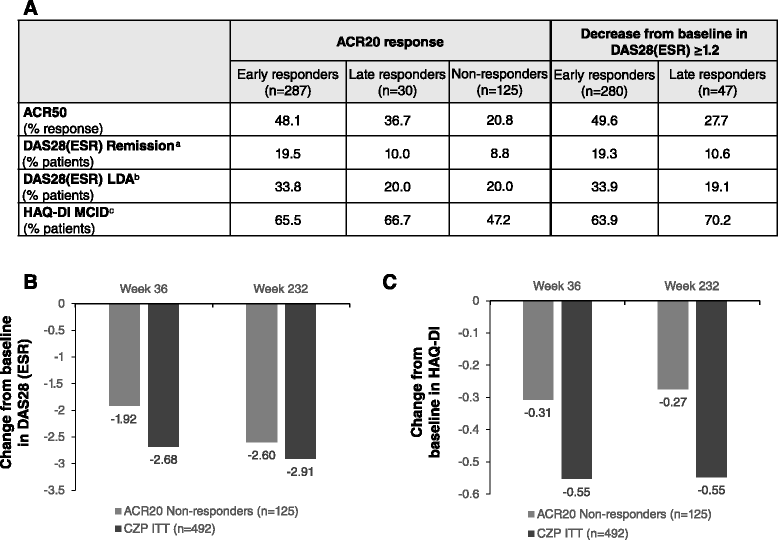
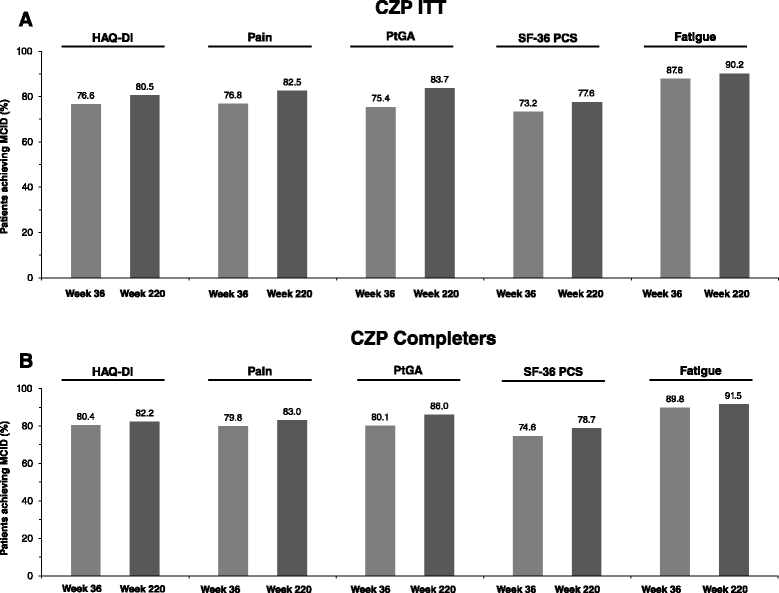
Results: In the RCT, 619 patients were randomized to CZP+MTX (n=492) or placebo+MTX (n=127). Overall, 567 patients (91.6%) entered the OLE: 447 CZP and 120 placebo patients. Of all randomized patients, 358 (57.8%) were ongoing at Week 232. Annual drop-out rates during the first four years ranged from 8.4-15.0%. Event rates per 100 patient-years were 163.0 for adverse events (AEs) and 15.7 for serious AEs. Nineteen patients (3.1%) had fatal AEs (incidence rate=0.8). Clinical improvements in the RCT were maintained to Week 232 in the CZP Completers: mean Disease Activity Score 28 (Erythrocyte Sedimentation Rate) change from baseline was -3.4 and ACR20/50/70 responses 68.4%/47.1%/25.1% (non-responder imputation). Similar improvements observed in the ITT were maintained following dose-reduction. 73.2% of CZP Completers had no radiographic progression at Week 128.
Conclusions: In patients with active RA despite MTX therapy, CZP was well tolerated, with no new safety signals identified. CZP provided sustained improvements in clinical outcomes for almost 5 years.
Trial registration: ClinicalTrials.gov, NCT00160602 and NCT00160641 . Registered 8 September 2005.
Figures


References
-
- Keystone E, Heijde D, Mason Jr D, Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–29. - PubMed
-
- van Riel PL, van Gestel AM, van de Putte LB. Development and validation of response criteria in rheumatoid arthritis: steps towards an international consensus on prognostic markers. Br J Rheumatol. 1996;35:4–7. - PubMed
-
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

