Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes
- PMID: 26354121
- PMCID: PMC4586885
- DOI: 10.1073/pnas.1516237112
Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes
Abstract
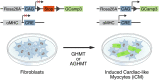
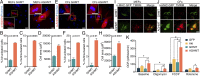
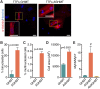
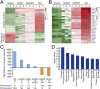
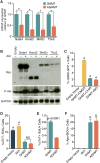
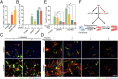
Conversion of fibroblasts to functional cardiomyocytes represents a potential approach for restoring cardiac function after myocardial injury, but the technique thus far has been slow and inefficient. To improve the efficiency of reprogramming fibroblasts to cardiac-like myocytes (iCMs) by cardiac transcription factors [Gata4, Hand2, Mef2c, and Tbx5 (GHMT)], we screened 192 protein kinases and discovered that Akt/protein kinase B dramatically accelerates and amplifies this process in three different types of fibroblasts (mouse embryo, adult cardiac, and tail tip). Approximately 50% of reprogrammed mouse embryo fibroblasts displayed spontaneous beating after 3 wk of induction by Akt plus GHMT. Furthermore, addition of Akt1 to GHMT evoked a more mature cardiac phenotype for iCMs, as seen by enhanced polynucleation, cellular hypertrophy, gene expression, and metabolic reprogramming. Insulin-like growth factor 1 (IGF1) and phosphoinositol 3-kinase (PI3K) acted upstream of Akt whereas the mitochondrial target of rapamycin complex 1 (mTORC1) and forkhead box o3 (Foxo3a) acted downstream of Akt to influence fibroblast-to-cardiomyocyte reprogramming. These findings provide insights into the molecular basis of cardiac reprogramming and represent an important step toward further application of this technique.
Keywords: cardiogenesis; cardiomyopathy; heart; regeneration; transdifferentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
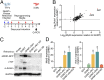
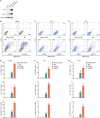
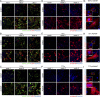
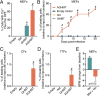

Comment in
-
Repairing hearts with AKT.Proc Natl Acad Sci U S A. 2015 Oct 27;112(43):13131-2. doi: 10.1073/pnas.1517489112. Epub 2015 Oct 19. Proc Natl Acad Sci U S A. 2015. PMID: 26483486 Free PMC article. No abstract available.
-
Understanding Intracellular Signaling Advances Cardiac Reprogramming Technology Toward Clinical Applications.Circ Res. 2016 Feb 5;118(3):377-8. doi: 10.1161/CIRCRESAHA.115.308075. Circ Res. 2016. PMID: 26846636 No abstract available.
References
-
- Inagawa K, et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111(9):1147–1156. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

