Optogenetic tools for modulating and probing the epileptic network
- PMID: 26354163
- PMCID: PMC4567692
- DOI: 10.1016/j.eplepsyres.2015.06.010
Optogenetic tools for modulating and probing the epileptic network
Abstract
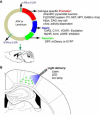
Epilepsy affects roughly 1% of the population worldwide. Although effective treatments with antiepileptic drugs are available, more than 20% of patients have seizures that are refractory to medical therapy and many patients experience adverse effects. Hence, there is a continued need for novel therapies for those patients. A new technique called "optogenetics" may offer a new hope for these refractory patients. Optogenetics is a technology based on the combination of optics and genetics, which can control or record neural activity with light. Following delivery of light-sensitive opsin genes such as channelrhodopsin-2 (ChR2), halorhodopsin (NpHR), and others into brain, excitation or inhibition of specific neurons in precise brain areas can be controlled by illumination at different wavelengths with very high temporal and spatial resolution. Neuromodulation with the optogenetics toolbox have already been shown to be effective at treating seizures in animal models of epilepsy. This review will outline the most recent advances in epilepsy research with optogenetic techniques and discuss how this technology can contribute to our understanding and treatment of epilepsy in the future.
Keywords: Epilepsy model; Optical imaging; Optogenetic; Photostimulation.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



References
-
- Alford SC, Wu J, Zhao Y, Campbell RE, Knöpfel T. Optogenetic reporters. Biology of the Cell. 2013;105:14–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

