Efficacy of stem cell in improvement of left ventricular function in acute myocardial infarction--MI3 Trial
- PMID: 26354213
- PMCID: PMC4613437
- DOI: 10.4103/0971-5916.164245
Efficacy of stem cell in improvement of left ventricular function in acute myocardial infarction--MI3 Trial
Abstract
Background & objectives: Acute myocardial infarction (AMI) is characterized by irreparable and irreversible loss of cardiac myocytes. Despite major advances in the management of AMI, a large number of patients are left with reduced left ventricular ejection fraction (LVEF), which is a major determinant of short and long term morbidity and mortality. A review of 33 randomized control trials has shown varying improvement in left ventricular (LV) function in patients receiving stem cells compared to standard medical therapy. Most trials had small sample size and were underpowered. This phase III prospective, open labelled, randomized multicenteric trial was undertaken to evaluate the efficacy in improving the LVEF over a period of six months, after injecting a predefined dose of 5-10 × 10 [8] autologous mononuclear cells (MNC) by intra-coronary route, in patients, one to three weeks post ST elevation AMI, in addition to the standard medical therapy.
Methods: In this phase III prospective, multicentric trial 250 patients with AMI were included and randomized into stem cell therapy (SCT) and non SCT groups. All patients were followed up for six months. Patients with AMI having left ventricular ejection fraction (LVEF) of 20-50 per cent were included and were randomized to receive intracoronary stem cell infusion after successfully completing percutaneous coronary intervention (PCI).
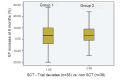
Results: On intention-to-treat analysis the infusion of MNCs had no positive impact on LVEF improvement of ≥ 5 per cent. The improvement in LVEF after six months was 5.17 ± 8.90 per cent in non SCT group and 4.82 ± 10.32 per cent in SCT group. The adverse effects were comparable in both the groups. On post hoc analysis it was noted that the cell dose had a positive impact when infused in the dose of ≥ 5 X 10 [8] (n=71). This benefit was noted upto three weeks post AMI. There were 38 trial deviates in the SCT group which was a limitation of the study.
Interpretation & conclusions: Infusion of stem cells was found to have no benefit in ST elevation AMI. However, the procedure was safe. A possible benefit was seen when the predefined cell dose was administered which was noted upto three weeks post AMI, but this was not significant and needs confirmation by larger trials.
Conflict of interest statement
Figures




References
-
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. - PubMed
-
- Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7:119–25. - PubMed
-
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–5. - PubMed
-
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348:771–5. - PubMed
-
- Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS, et al. CREATE registry investigators. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–42. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
