Afatinib in Non-Small Cell Lung Cancer Harboring Uncommon EGFR Mutations Pretreated With Reversible EGFR Inhibitors
- PMID: 26354527
- PMCID: PMC4591955
- DOI: 10.1634/theoncologist.2015-0073
Afatinib in Non-Small Cell Lung Cancer Harboring Uncommon EGFR Mutations Pretreated With Reversible EGFR Inhibitors
Abstract
Background: Afatinib, an irreversible ErbB family blocker, is approved for treatment of patients with previously untreated non-small cell lung cancer (NSCLC) harboring activating epidermal growth factor receptor (EGFR) mutations. Efficacy of afatinib in EGFR tyrosine kinase inhibitor-naïve (TKI-naïve) patients with uncommon EGFR mutations (other than exon 19 deletions or exon 21 point mutations) has been reported; however, efficacy in TKI-pretreated patients with uncommon EGFR mutations is unknown.
Materials and methods: In the afatinib compassionate use program (CUP), patients with advanced or metastatic, histologically confirmed NSCLC progressing after at least one line of chemotherapy and one line of EGFR-TKI treatment were enrolled. Demographic data, mutation type, response rates, time to treatment failure (TTF), and safety in patients harboring uncommon EGFR mutations were reported.
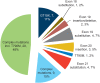
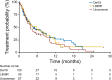
Results: In 60 patients (63% female, median age 63 years [range: 30-84 years]), a total of 66 uncommon EGFR mutations including 30 T790M mutations were reported (18.4% and 11%, respectively, of known EGFR mutations within the CUP). Most patients (67%) received afatinib as third- or fourth-line treatment. Median TTF was 3.8 months (range: 0.2 to >24.6 months; p = .244) in patients with uncommon mutations compared with 5.1 months (range: 0.1 to >21.1 months) in patients with common mutations (n = 165). Pronounced activity was observed with E709X mutations (TTF >12 months). No new safety signals were detected.
Conclusion: Afatinib is clinically active and well tolerated in many TKI-pretreated NSCLC patients harboring uncommon EGFR mutations. Compared with results reported in TKI-naïve patients, activity was also indicated in patients with T790M and exon 20 insertion mutations.
Keywords: Afatinib; Compassionate use trials; Epidermal growth factor receptor; ErbB receptors; Non-small cell lung cancer.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: First line or second line--is there a difference? J Clin Oncol. 2013;31:1081–1088. - PubMed
-
- Rosell R, Molina MA, Costa C, et al. Outcome to erlotinib in non-small cell lung cancer (NSCLC) patients (p) according to the presence of the EGFR T790M mutation and BRCA1 mRNA expression levels in pretreatment biopsies. J Clin Oncol. 2010;28:7514a.
-
- Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343:342–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous